- Policy
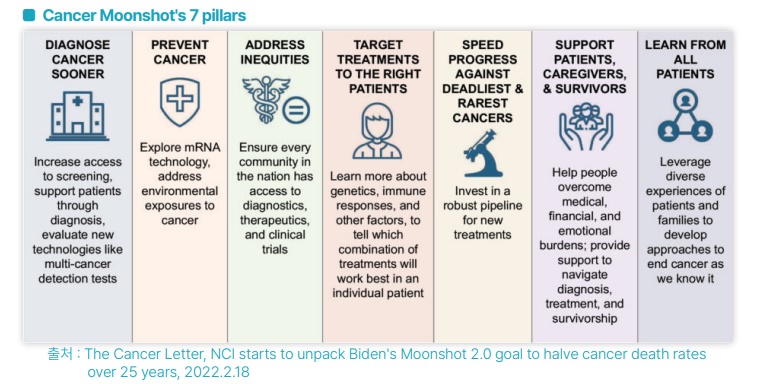
- ‘Introduce a Korean Cancer Moonshot Plan for patients'
- by Lee, Jeong-Hwan Sep 21, 2023 05:24am
- It has been pointed out that Korea also needs a policy that can improve the quality of life of cancer patients by providing reasonable compensation for cancer treatment technologies such as robot-assisted surgery and advanced radiation therapy to improve the quality of life of cancer patients in Korea, like in the case of the U.S. Cancer Mo
- Opinion
- [Reporter’s View] Biobetters to prosper with better prices
- by Eo, Yun-Ho Sep 21, 2023 05:23am
- A series of cases where preferential pricing has been applied to biobetter drugs are being introduced to Korea. In 2016, the government announced a plan to provide preferential pricing for biosimilars and biobetters, which are improved versions of already approved biopharmaceuticals, that have contributed to the improvement of Korea’s healt
- Policy
- Generics for Janumet competition begins in earnest in Oct.
- by Lee, Tak-Sun Sep 21, 2023 05:23am
- The diabetes treatment market is expected to remain active in October. This is because salt-modifying complex drugs that were not registered in September are hitting the market all at once. According to the industry on the 20th, generics of MSD's DPP-4 inhibitory diabetes combination drug 'Janumet and Janumet XR' will be listed in large numb
- Opinion
- [Reporter’s View] Industry suffers from ‘Re-eval Neurosis'
- by Kim, Jin-Gu Sep 20, 2023 05:36am
- The era of reevaluations has dawned on Korea. Reevaluation of reimbursement adequacy, clinical reevaluations, and generic drug pricing reevaluations are being carried out simultaneously. The Ministry of Health and Welfare and its affiliated organizations appear to be scrambling to reevaluate and reduce drug pricing expenditures as if they ha
- Policy
- High-priced drugs need adjustment from the approval stage
- by Lee, Tak-Sun Sep 20, 2023 05:36am
- He also explained that he would conduct a pilot project to curb multi-drug prescriptions to reduce usage. Chairman Jeong made this announcement at a press conference for a health magazine held at a restaurant in Gwanghwamun, Seoul on the 15th. He said, “It is true that as income increases, prescriptions switch to more expensive drugs even i
- Policy
- The NHIS begins negotiations to increase the size of PSE
- by Lee, Tak-Sun Sep 20, 2023 05:36am
- The NHIS has started negotiations to adjust the drug price increase for the ingredient formulation of the nose cold medicine Pseudoephedrine (PSE), which is struggling with supply and demand. The deadline for this negotiation is 60 days, but it is said to be targeting an agreement this month. If this month's negotiations are signed, it is h
- Company
- A new drug for optic shylomyelitis is necessary
- by Eo, Yun-Ho Sep 20, 2023 05:35am
- Off labels refer to the act of prescribing a medicine for an indication other than a use approved by the Ministry of Food and Drug Safety. In general, the use of the drug has been determined by the health authorities, and the question may arise as to why it is necessary. However, there is an area of disease where this Off label prescri
- Policy
- Reimbursement imminent for some anticancer drugs in KOR
- by Lee, Tak-Sun Sep 20, 2023 05:35am
- Jeperli Inj (dostarlimab, GSK) and Braftovi Cap 75mg (encorafenib, Ono), which passed the Drug Reimbursement Evaluation Committee meeting last month, are currently in drug price negotiations with the National Health Insurance Service. According to industry sources on the 19th, the National Health Insurance Service recently updated the li
- Company
- Pricing & reimbursement specialist introduced as a new job
- by Kang, Shin-Kook Sep 20, 2023 05:35am
- New occupations such as ‘Pricing & reimbursement specialist’ and ‘biopharmaceutical candidate substance researcher’ were added as new occupations in the bio and pharmaceutical industry. On the 19th, the Korea Employment Information Service (President Young-Jun Kim) announced it had added 156 new occupations in the fields of life scien
- Company
- No news after passing the cancer screening
- by Eo, Yun-Ho Sep 20, 2023 05:35am
- Xospata's progress in expanding insurance benefits, which seemed to be going smoothly, appears to have stopped. According to related industries, Astellas Korea Pharmaceutical's FLT3 mutation-positive recurrence or Acute Myeloid Leukemia treatment Xospata was not submitted during the three HIRA Pharmaceutical Reimbursement Evaluation Commi