- Company
- Korea United Pharm signs export agreement with Vietnam TAK
- by Nho, Byung Chul Sep 19, 2023 05:42am
- On the 18th, Korea United Pharm (CEO Duk-Young Kang) attended the 2023 Global Bio& Pharma Plaza (GBPP) 2023 and forged close relationships with over 20 overseas buyers. The company announced that it had accelerated its global expansion by signing export contracts for Vietnam and the Philippines on the day of the event. Korea United Phar
- Policy
- MFDS starts review on phenylephrine after FDA advisory decis
- by Lee, Hye-Kyung Sep 19, 2023 05:42am
- With the Nonprescription Drug Advisory Committee (NDAC) concluding that the nasal decongestant ‘phenylephrine’ is not effective, Korea’s Ministry of Food and Drug Safety is also preparing to conduct its review. An MFDS official said, “We will decide on future measures after a comprehensive review, including discussion with experts,
- Policy
- Januvia generics up for insurance price cuts… 119 combinati
- by Lee, Tak-Sun Sep 19, 2023 05:41am
- Caution is required for the use of the generic versions CPP-4 inhibitor class antidiabetic drug ‘Januvia (sitagliptin, MSD)’ that have poured out into the Korean market as many are subject to price cuts due to inequality of price when they are prescribed in multiplications. According to industry sources on the 18th, HIRA recently added
- Policy
- Olumiant re-examination period extended by 35 months
- by Lee, Hye-Kyung Sep 18, 2023 05:26am
- In March of this year, the post-sales investigation (PMS) plan of Lilly Korea Olumiant, which was approved for severe circular alopecia indications in adult patients over the age of 18 from the Ministry of Food and Drug Safety in March of this year, will also be changed. According to the minutes of the meeting of the Central Pharmaceutical R
- Company
- nAMD drug Vabysmo will likely be listed for reimb in Oct
- by Eo, Yun-Ho Sep 18, 2023 05:26am
- The macular degeneration treatment ‘Vabysmo’ is expected to be listed for reimbursement in October this year. According to industry sources, Roche Korea has completed drug pricing negotiations for its bispecific antibody Vabysmo (faricimab) with the National Health Insurance Service. Once it passes the Health Insurance Policy Review Com
- Policy
- Super-antibiotic Tygacil reauthorized for use in Korea
- by Lee, Tak-Sun Sep 18, 2023 05:26am
- The ‘super-antibiotic’ Tygacil (Tigecycline, Pfizer Korea) received reauthorization in Korea and is expected to be able to avoid the treatment disruptions that had remained a lingering concern in the industry. Due to the lack of other drugs containing the same ingredient, there had been concerns that Tygacil’s failed renewal would cau
- Company
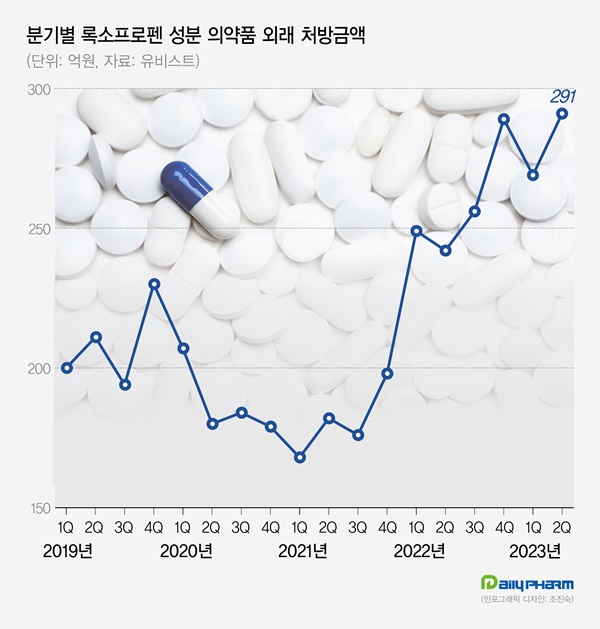
- KRW 100 bil loxoprofen market suffers reimb cuts
- by Chon, Seung-Hyun Sep 18, 2023 05:26am
- Industry concerns are rising on the announcement of reimbursement cuts for the nonsteroidal anti-inflammatory drug ‘loxoprofen.’ The adequacy of its reimbursement as an antipyretic/analgesic for acute upper respiratory tract inflammation has not been recognized by the authorities in Korea, and its scope of prescriptions is set to be narrowed a
- Policy
- Authorization rate for No.16 is still 0%
- by Lee, Hye-Kyung Sep 18, 2023 05:26am
- While the Ministry of Food and Drug Safety recently completed the designation of 'Global Innovative Product Rapid Review (GIFT)' No. 16, there are still no items that have been approved using this system. The Ministry of Food and Drug Safety established the Global Innovative Products on Fast Track in September last year. It also contains innova
- Policy
- Celltrion receives approval for Nesina Met SR
- by Lee, Hye-Kyung Sep 15, 2023 05:33am
- Celltrion, which acquired the Asia-Pacific distribution rights for Takeda Pharmaceutical products, used them to create an improved new drug. One of the acquired products, Nesina Met SR, a type 2 diabetes treatment, received approval from the Ministry of Food and Drug Safety. The Ministry of Food and Drug Safety announced on the 14th that
- Policy
- Which P-CAB generic will come first, K-CAB or Vocinti?
- by Lee, Tak-Sun Sep 15, 2023 05:33am
- Companies have started developing generic versions of the P-CAB (potassium-competitive acid blocker) class gastroesophageal reflux disease (GERD) treatments K-CAB (tegoprazan, HK Inno.N) and Vocinti (vonoprazan, Takeda Pharmaceuticals Korea). The post-marketing surveillance (PMS) period for the original drugs, which are being targeted