- Company
- Leclaza’s cumulative sales reach KRW 30 bil in 2 yrs
- by Chon, Seung-Hyun Aug 30, 2023 05:32am
- Yuhan Corp’s new anticancer drug, ‘Leclaza’ is gradually expanding its influence in the domestic market. The drug exceeded KRW 30 billion in cumulative sales in only 2 years since its domestic release. Prescriptions are also expected to increase further if the company receives reimbursement for its newly added indication as a first-line treat
- Company
- Sales of Taxol & Genexol rise together
- by Kim, Jin-Gu Aug 30, 2023 05:31am
- Sales of Taxol and Genexol, which contain paclitaxel, rose together in 1H this year. After the sales companies for the two drugs were switched, competition between Boryeong, which has Taxol, and HK Inno.N, which owns Genexol, have been intensifying. According to the industry research institution IQVIA on the 30th, Taxol posted sales of
- Company
- Perjeta and Herceptin combination drug, Phesgo
- by Eo, Yun-Ho Aug 30, 2023 05:31am
- Phesgo, a subcutaneous injection-type combination of Perjeta and Herceptin, challenges insurance coverage registration. As a result of the coverage, Phesgo of Roche Korea is presented to the Cancer Disease Review Committee of the HIRA today (30th). This is the first case of an improved anti-cancer biobetter. Biobetter refers to drugs reco
- Company
- Keytruda posts sales of KRW 183.8 bil in 1H...unrivaled lead
- by Chon, Seung-Hyun Aug 29, 2023 05:28am
- The immuno-oncology drug Keytruda’s sales have been skyrocketing in the domestic drug market. In the first half of the year alone, the drug posted sales of over KRW 200 billion and secured its unrivaled lead in the market. The drug’s sales have been rising more steeply after the drug’s reimbursement was extended as first-line therapy last yea
- Policy
- Opdivo reimb as first-line therapy in gastric cancer in KOR
- by Lee, Tak-Sun Aug 29, 2023 05:28am
- The immuno-oncology drug Opdivo will be available for use as a first-line treatment, and the administration subjects for Imbruvica and G-CSF injections will be expanded. The Health Insurance Review and Assessment Service started collecting opinions on the amendment of the reimbursement standards that contain the changes above from the 24th.
- Policy
- Hyundai Pharm's Hypejil 3mg
- by Lee, Tak-Sun Aug 29, 2023 05:28am
- A 3mg low-dose product is listed for the first time in a Donepezil tablet used to treat Alzheimer's. The main character is Hyundai Pharm's 'Hypejil Tablet 3mg'. This drug will be paid from September to 486 won for the party according to the calculation criteria. According to the industry on the 25th, 3 mg of Donepezil is the first time
- Company
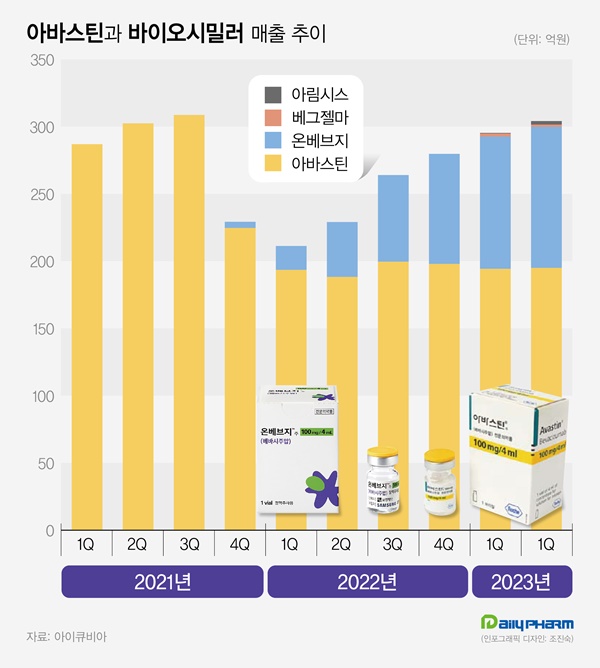
- The synergy between technology and sales force
- by Chon, Seung-Hyun Aug 29, 2023 05:28am
- Domestically developed biosimilar products are prominent in the large anticancer drug Asastin market. Samsungbioepis' Onbevzi exceeded 10 billion won in quarterly sales in the first two years of its release, showing a 35% share. It is said that Boryung, which was the first biosimilar to enter the market and has differentiated strengths in antica
- Company
- Verquvo busy securing prescriptions in KOR with reimb
- by Eo, Yun-Ho Aug 29, 2023 05:28am
- The new heart failure drug ‘Verquvo’ is busy securing prescriptions in Korea with its reimbursement listing. According to industry sources, Bayer Korea’s soluble Guanylate Cyclase (sGC) stimulator Verquvo (Vericiguat)’ passed the drug committees of Korea Anam Hospital, Chung-Ang University Gwangmyeong Hospital, Seoul National Universi
- Policy
- MOHW-Roche have difficulty discussing resupply of Madopar
- by Lee, Jeong-Hwan Aug 28, 2023 05:21am
- The Ministry of Health and Welfare seems to be having difficulty bringing in Madopar, a Parkinson's disease drug that had withdrawn from the domestic market, back to Korea. The MOHW had previously announced that it would make efforts to resupply Madopar in Korea. Roche Korea is known to have refused to supply Madopar Tab to Korea, citing
- Company
- Price negotiation period for Spinraza and Evrysdi extended
- by Eo, Yun-Ho Aug 28, 2023 05:21am
- The companies of the spinal muscular atrophy (SMA) treatments ‘Spinraza’ and ‘Evrysdi’ failed to conclude drug pricing negotiations for their drugs within the deadline and began extended negotiations. As a result, Spinraza reimbursement extension and Evrysdi’s new listing will only be possible in October at the earliest. Accordi