- Company
- Janssen, Pharma distribution industry agree on margin adj
- by Son, Hyung Min Aug 1, 2025 06:18am
- The conflict between Janssen Korea and the pharmaceutical distribution industry over distribution margin reductions has been resolved with a negotiated settlement. The distribution industry achieved an outcome that maintains existing trade relationships, including with small and medium-sized distributors, while also improving the proposed mar
- Policy
- ENT and pediatric departments prescribe the most drugs
- by Lee, Hye-Kyung Aug 1, 2025 06:16am
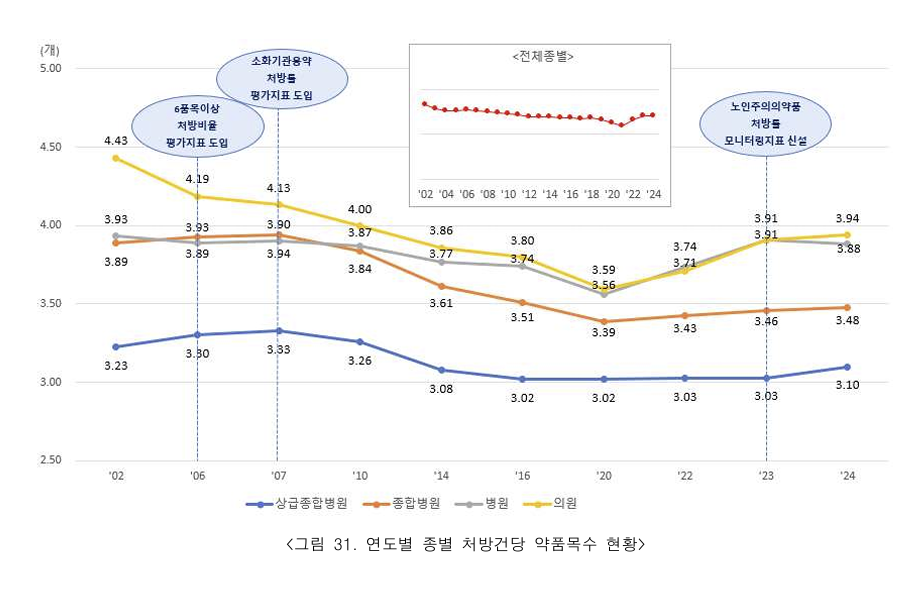
- Last year, medical institutions prescribed an average of 3.85 drugs per prescription. Outpatient clinics prescribed the most at 3.94, followed by hospitals at 3.88, general hospitals at 3.48, and tertiary hospitals at 3.10. The percentage of medical institutions prescribing 6 or more drugs increased by 0.79% from 18.34% in the previous year t
- Policy
- PPP also proposes free HPV vaccination for ppl under 26
- by Lee, Jeong-Hwan Aug 1, 2025 06:16am
- Following the ruling party, the main opposition party, the People Power Party, also submitted a bill to the National Assembly to provide free HPV vaccines to all citizens aged 26 and under, regardless of gender. The People Power Party’s bill also included provisions to expand the scope of free influenza vaccinations beyond the current co
- Company
- NMOSD drug Enspryng’s reimbursement expanded in KOR
- by Whang, byung-woo Aug 1, 2025 06:16am
- Roche Korea announced on the 31st that the reimbursement criteria for Enspryng (satralizumab), a treatment for neuromyelitis optica spectrum disorder (NMOSD), will be expanded from August 1st per a notification from the Ministry of Health and Welfare. Enspryng is indicated for the treatment of adult patients with anti-aquaporin(AQP4) anti
- Opinion
- [Reporter's View] KRPIA opts for 'maintain' over 'change'
- by Eo, Yun-Ho Aug 1, 2025 06:15am
- The Korea Research-based Pharma Industry Association (KRPIA) has chosen continuity over change. The KRPIA recently confirmed the appointment of Vice Chairman Lee Young-shin (68) to the position of Executive Vice Chairman, who is currently leading the association. This decision was made after amending the articles of incorporation to addres
- Company
- Roche's HR+ breast cancer drug 'Itovebi' wins nod in Korea
- by Whang, byung-woo Aug 1, 2025 06:15am
- Roche Korea announced on July 30 that it has received approval of the breast cancer treatment Itovebi (inavolisib) from the Ministry of Food and Drug Safety. Itovebi, recently approved, can be used for the treatment of PIK3CA mutation-positive, hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) breas
- Company
- Beyfortus likely to face restrictions on public advertising
- by Whang, byung-woo Jul 31, 2025 06:15am
- Sanofi is now compelled to revise its strategy as public advertising of Beyfortus (nirsevimab), an RSV preventive antibody injection, faces regulatory obstacles in Korea. According to Dailypharm’s coverage, the&160;Korea Pharmaceutical and Bio-Pharma Manufacturers Association (KPBMA) Drug Advertising Review Committee ruled that Beyfortu
- Policy
- MOHW seeks measures to attract domestic investment
- by Lee, Jeong-Hwan Jul 31, 2025 06:15am
- The government is seeking ways to encourage multinational pharmaceutical companies to strengthen domestic investment, including the establishment of new pharmaceutical production plants in Korea. However, international trade issues and other obstacles remain to be overcome to attract domestic investment from multinational pharmaceutical co
- Company
- MSD Korea begins Januvia drug price difference compensation
- by Son, Hyung Min Jul 31, 2025 06:14am
- There are changes regarding the Januvia drug price difference compensation issue, which had been stalled for nearly two years. MSD Korea has officially informed the distribution industry that it will begin accepting applications for Januvia compensation starting next month, August 1. However, this compensation is limited to the quantity sold
- Company
- GSK Korea appoints Gunnar Riediger as new General Manager
- by Whang, byung-woo Jul 31, 2025 06:14am
- GSK Korea announced on the 30th that it has appointed Gunnar Riediger as its new General Manager, effective August 1. The new GM joined GSK in 2004 through the company's global talent development program, the “Future Leaders Program,” and has led healthcare operations across Latin America for over 20 years. He has held key roles incl