- Policy
- GPP drug Spevigo is first approved in Korea
- by Lee, Hye-Kyung Aug 11, 2023 05:36am
- The new orphan drug Spevigo (spesolimab) that is being imported by ‘Boehringer Ingelheim Korea has received domestic marketing authorization in Korea. The Ministry of Food and Drug Safety (Minister Yu-Kyung Oh) approved the new orphan drug Spevigo on the 9th. Generalized Pustular Psoriasis (GPP) is characterized by systemic inflamma
- Policy
- PVG-004 succeeded in securing BA as the 1st generic K-CAB
- by Lee, Jeong-Hwan Aug 11, 2023 05:35am
- On the 10th, Pharmavision announced that the PVG-004 (Tegoprazan) project, which had transferred technology to SCD, succeeded in securing equivalence in the K-CAB and BA test for the first time in Korea. The reexamination expiration date (PMS) for K-CAB is July 4, 2024. Substance patents expire on August 25, 2031, and crystalline patents expi
- Policy
- 'Apply preferential pricing to non-inferior new drugs'
- by Lee, Tak-Sun Aug 11, 2023 05:35am
- With the government planning to release a drug price reform plan that includes a fair value compensation plan for innovative new drugs as early as next month, attention is focused on whether the reform will include a preferential measure for new drugs that demonstrate non-inferiority. This is because most of the new drugs that domestic pharma
- Policy
- Health insurance finance approved by the government alone
- by Lee, Jeong-Hwan Aug 11, 2023 05:35am
- It was pointed out again that health insurance finance should be included in the national finance and operated as a fund. There are concerns that if the health insurance finances are managed without going through deliberation by the National Assembly as it is now, transparency will be reduced and it can lead to negligence. The current method
- Company
- Will Darzalex be reimb as 2nd-line treatment for MM?
- by Eo, Yun-Ho Aug 11, 2023 05:35am
- Whether progress will be made on the discussions on extending reimbursement of the multiple myeloma treatment ‘Darzalex’ as second-line treatment is gaining attention. According to industry sources, Janssen Korea is reattempting to apply for reimbursement of its 'Darzalex (daratumumab)' to be reviewed by the Health Insurance Review and
- Policy
- Out of stock of GLP-1 injections
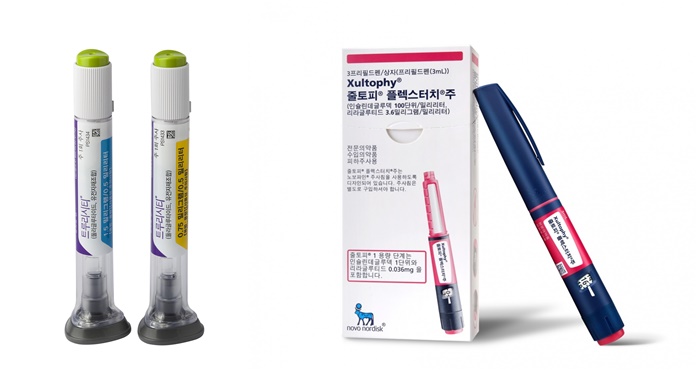
- by Moon, sung-ho Aug 10, 2023 05:33am
- As the news of GLP-1 (Glucagon-like peptide-1)-type diabetes treatments being used in clinical settings is reported one after another, concerns about this are growing. According to the medical community on the 4th, Novo Nordisk Pharmaceutical Korea is currently informing the clinical site of the out-of-stock news of Xultophy, a GLP-1 series diab
- Opinion
- [Reporter's view] Prompt benefit registration
- by Eo, Yun-Ho Aug 10, 2023 05:33am
- These days, the word 'expedited' appears frequently in the reform of the insurance benefit system. The plan is to speed up the listing of necessary new drugs by linking approval, and evaluation, and shortening the listing period at each stage. This is a good intention to quickly expand drug coverage for patients who are waiting. Shortening t
- Company
- Lotte Biologics advances CDMO Biz with Roche Diagnostics
- by Jung, Sae-Im Aug 10, 2023 05:33am
- On the 9th, Lotte Biologics announced that it had recently signed a Memorandum of Understanding (MOU) with Roche Diagnostics, the diagnostic division of the Roche Group, to advance the productivity and quality of its Contract Development and Manufacturing Organization (CDMO) business. The MOU, which was signed at Penzberg, where Roche Dia
- Company
- Generic drugs occupy over 70% of tamsulosin market
- by Kim, Jin-Gu Aug 10, 2023 05:33am
- The share of generic drugs in the market for prostatic hyperplasia treatments that contain tamsulosin exceeded 70%. This is interpreted as an effect of the rapid rise in generic sales while the prescription performance of original products slowed down. One variable in this market is impurities. At the end of June, impurities were detected i
- Company
- Patent dispute over Entresto's patents continue
- by Kim, Jin-Gu Aug 10, 2023 05:33am
- The patent dispute over ‘Entresto (sacubitril/valsartan),’ a chronic heart failure treatment with an annual prescription of more than KRW 40 billion, has been ongoing for over 2 years now. Novartis, the company that owns the original drug, is actively pursuing a defense strategy against generic companies' patent challenges. Novartis app