- Policy
- Incentives for phase 3 among Koreans raise public opinion
- by Nho, Byung Chul Aug 1, 2023 05:33am
- In order to prepare a reasonable drug price calculation for new drugs that have undergone phase 3 clinical trials in Korea, attention is focused on whether the special drug price system of Japan, Taiwan, and France can be applied and introduced. According to the industry, the public-private consultative body for improving the drug price syst
- Policy
- Decided to re-discuss reimbursement criteria for Mylotarg
- by Lee, Tak-Sun Jul 31, 2023 05:29am
- Acute myeloid leukemia treatment drug Mylotarg decided to re-discuss setting reimbursement standards. This drug received attention as the first Antibody-Drug Conjugate (ADC) treatment, but in May of last year, the Cancer Disease Review Committee failed to establish reimbursement standards. The HIRA (Chief Director Kang Jung-gu) announced that it
- Opinion
- [Reporter's view] When will Enhertu's benefit be available?
- by Jul 31, 2023 05:29am
- Daiichi Sankyo and AstraZeneca's Enhertu are continuing their unstoppable moves. Enhertu, which has achieved remarkable results in breast cancer, has expanded the types of carcinoma to include gastric cancer and lung cancer and is expected to be used in a number of solid cancers that show HER2 expression, such as cervical cancer, endometrial
- Policy
- Bill proposed to allow Kymriah’s use at transplant hospital
- by Kim, Jung-Ju Jul 31, 2023 05:28am
- A bill was proposed to amend the law and improve patient access to Novartis’s Kymirah, the ultra-high-priced ‘one-shot drug,’ by realistically amending the requirements for institutions that use the drug. The main point of the amendment is to allow hematopoietic stem cell transplant institutions designated as transplant hospitals to pr
- Company
- Equipped with two new drugs this year alone
- by Jul 31, 2023 05:28am
- Janssen challenges with a new mechanism, BMS widening the gap with maintenance therapy Janssen has added a new drug for multiple myeloma in Korea. This is the second permit this year. It is noteworthy whether Janssen will change the multiple myeloma market where BMS dominates with Celgene. On the 26th, the Ministry of Food and Drug Safety ap
- Company
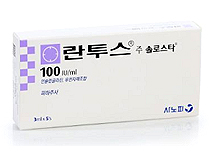
- Handok will sell original insulin drug Lantus
- by Lee, Tak-Sun Jul 31, 2023 05:28am
- Handok, which had previously stopped selling the insulin biosimilar ‘Glarzia (insulin glargine)', will be marketing and distributing the original insulin 'Lantus' from the 1st of next month. Whether the company will be able to expand its presence in the diabetes treatment market with the original product based on the know-how in insulin
- Company
- Reimb of Bayer’s new heart failure drug Verquvo imminent
- by Eo, Yun-Ho Jul 31, 2023 05:28am
- The new heart failure drug ‘Verquvo’ is expected to be reimbursed in Korea soon. According to industry sources, Bayer Korea’s soluble Guanylate Cyclase (sGC) stimulator ‘Verquvo (Vericiguat)’ has essentially completed drug pricing negotiations with the National Health Insurance Service. Therefore, the drug is expected to be listed fo
- Policy
- Re-evaluation of the upper limit amt, scheduled for August
- by Lee, Tak-Sun Jul 31, 2023 05:27am
- The final result of the re-evaluation of the upper limit amount standard requirement will be presented as an agenda for the Pharmaceutical Reimbursement Evaluation Committee to be held on August 3. It is expected that negotiations with NHIS will proceed in earnest after the submission of the committee. The results of the re-evaluation of the
- Company
- Erleada can be prescribed at tertiary hospitals in KOR
- by Eo, Yun-Ho Jul 28, 2023 05:30am
- The new prostate cancer drug Erleada has landed in general hospitals in Korea. According to industry sources, Janssen Korea’s metastatic hormone-sensitive prostate cancer (mHSPC) treatment Erleada (apalutamide) has passed the drug committee (DC) review at the Big 5 tertiary hospitals in Korea, - Samsung Medical Center, Seoul National Uni
- Policy
- Janssen’s MM drug Tecvayli Inj is approved in KOR
- by Lee, Hye-Kyung Jul 28, 2023 05:30am
- On the 26th, the Ministry of Food and Drug Safety (Minister: Yu-Kyung Oh) approved ‘Tecvayli Inj (teclistamab),’ a rare disease drug used to treat multiple myeloma. Tecvayli is used in adult patients with relapsed and refractory multiple myeloma who have received at least three previous lines of treatment, including a proteasome inhibit