- Company
- AZ and SK's Sidapvia is approved, first collab in 10 yrs
- by Jung, Sae-Im Jul 20, 2023 05:34am
- The first collaboration between a domestic company and big pharma has come to fruition. This is the first collaboration made in almost a decade since the development of Rovelito. Whether AstraZeneca, which is trying to increase the use of its diabetes treatment 'Forxiga' in clinics in Korea, and SK Chemicals, which is trying to increase producti
- Policy
- MFDS organizes a Clinical Trial Consultative Body
- by Lee, Tak-Sun Jul 19, 2023 05:20am
- The Ministry of Food and Drug Safety announced on the 17th that it has organized and is operating a ‘Clinical Trial Consultative Body’ with the industry to hold an ear out to the domestic clinical trial industry’s opinion and facilitate the smooth operation of the systems that are being newly introduced. The consultative body will con
- Policy
- 22 items that exploded in use during COVID-19,
- by Lee, Tak-Sun Jul 19, 2023 05:20am
- While negotiations are underway for PVA Type Da items, 22 items are said to have undergone correction due to increased usage due to Corona 19 last year. The NHIS plans to finalize the negotiations by this month, including these items, and report the results of the negotiations to the Health Insurance Policy Deliberation Committee of the Minis
- Policy
- No need to fear for the lack of Sabril after recall
- by Lee, Hye-Kyung Jul 19, 2023 05:20am
- The supply of Handok’s ‘Sabril Tab’ that was recalled by the company is expected to smoothen up soon. Its recall was issued by the health authorities due to the discovery of a small amount of an active pharmaceutical ingredient for a different drug, ‘tiapride,’ in its main active pharmaceutical ingredient, ‘vigabatrin.’ The Minist
- Opinion
- [Reporter's view]Time to think about price by indication
- by Eo, Yun-Ho Jul 19, 2023 05:20am
- Now is the time to think. The increasing number of non-insured indications and the steadily increasing number of indications for new drugs have now become quite a big snowball. In an age when a single drug has multiple indications and is used for multiple diseases, the emergence of drugs that target specific gene mutations and further activat
- Company
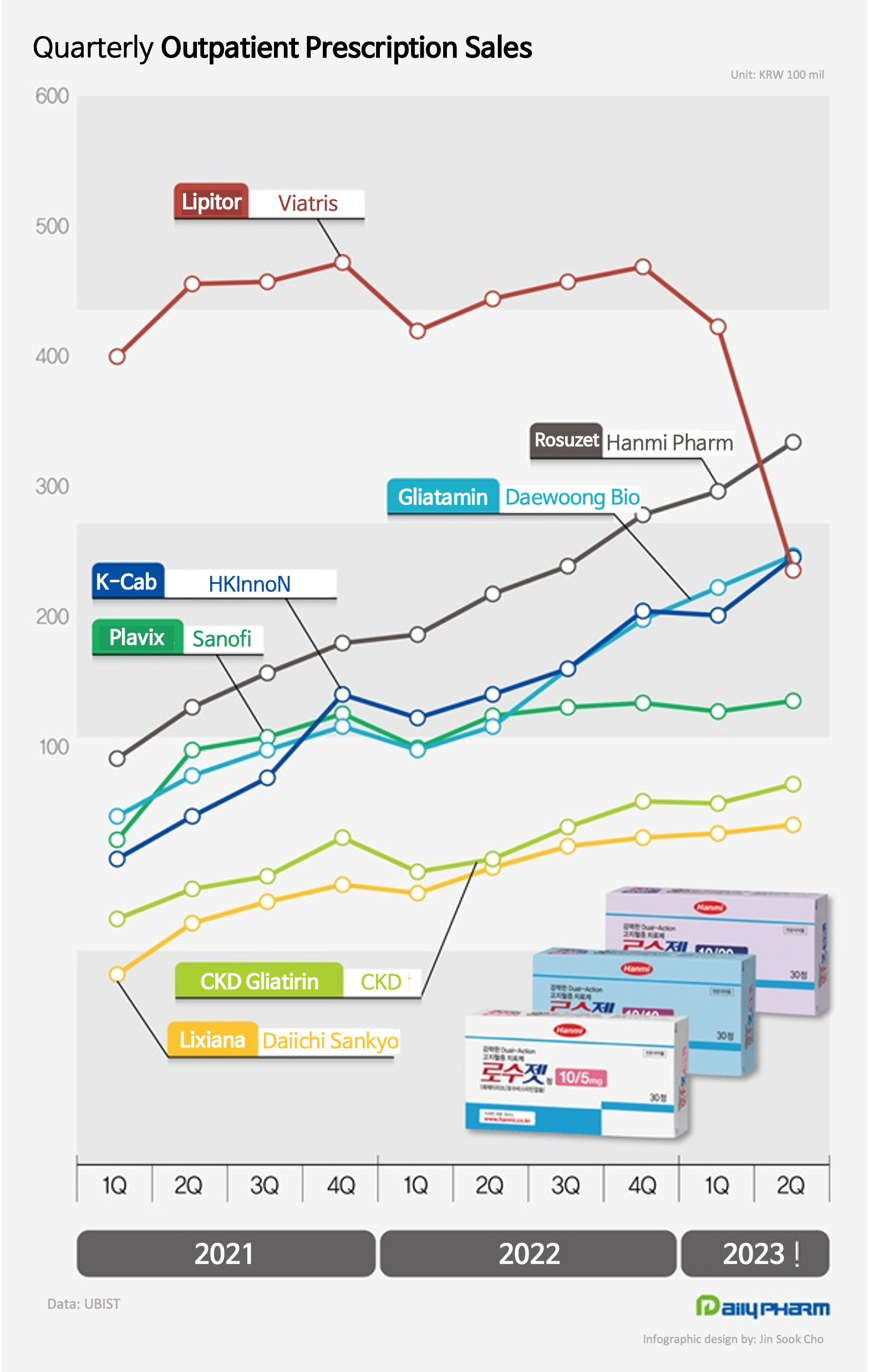
- Power of K-combos...Rosuzet leads outpatient Rx drug market
- by Chon, Seung-Hyun Jul 19, 2023 05:20am
- Hanmi Pharmaceutical’s new combination drug Rosuzet is ruling the outpatient prescription market in Korea. Rosuzet became the first domestically developed drug to lead quarterly outpatient prescriptions sales. Sales of HK Inno.N’s new drug ‘K-cab’ also continued to grow, taking part in the lead owned by domestically developed drugs in the ou
- Company
- Will GC Pharma enter the US blood product market in 2013?
- by Chon, Seung-Hyun Jul 19, 2023 12:28am
- GC Pharma has challenged the US immunoglobulin blood product market worth 13 trillion won. For the past 13 years since it officially entered the US market in 2010, it has experienced growing pains such as failure to obtain permits and delays, but has attempted to enter the US market again. According to the industry on the 18th, GC Pharma sub
- Company
- GC Pharma, reapplying to FDA for approval of immunoglobulin
- by Chon, Seung-Hyun Jul 19, 2023 12:20am
- GC Pharma planned to enter the US market first with 5% products and later with 10% products undergoing clinical trials. However, as the approval of the 5% product was delayed, the company changed its strategy to release the 10% product, which has greater marketability, to the US market first. GC Pharma completed phase 3 clinical trials
- Company
- FDA accepts NDA for HLB’s rivoceranib
- by Lee, Seok-Jun Jul 18, 2023 05:29am
- HLB’s hepatocellular carcinoma treatment ‘rivoceranib’ has now entered FDA’s review. Yang-Gon Jin, Chairman of the HLB Group, announced on the morning of the 17th that “HLB’s US subsidiary Elevar received an ‘NDA Filing Acceptance’ letter from the FDA.” Elevar submitted a new drug application (NDA) for rivoceranib on May 16 aft
- Company
- Boehringer Ingelheim releases SGLT2+DPP4 combo Esgliteo
- by Jung, Sae-Im Jul 18, 2023 05:29am
- On the 17th, Boehringer Ingelheim announced it had launched its type 2 diabetes treatment ‘Esgliteo (empagliflozin+linagliptin)’ in Korea. Esgliteo is a fixed-dose combination of Boehringer Ingelheim’s original SGLT-2 inhibitor ‘Jardiance (empagliflozin)’ and DPP-4 inhibitor ‘Trajenta (linagliptin). The company developed a small-si