- Company
- Discussions on expanding benefit for Jakavi's GVHD are slow
- by Eo, Yun-Ho Jun 13, 2023 05:43am
- Discussions on expanding insurance coverage for Jakavi's Graft-versus-Host Disease (GvHD) indication have been sluggish. According to the industry, Novartis Korea Jakavi passed the harmaceutical Reimbursement Criteria Subcommittee of the Health Insurance Review and Assessment Service last February, but it has not been submitted to the Financi
- Company
- Severe asthma Tx Tezpire to soon be commercialized in Korea
- by Eo, Yun-Ho Jun 13, 2023 05:43am
- The new drug for severe asthma, ‘Tezpire' is soon to enter the Korean market. According to industry sources, AstraZeneca Korea has submitted an application for the approval of ‘Tezspire (tezepelumab)’ in Q2 to the Ministry of Food and Drug Safety and is receiving review. At this pace, Tezspire is expected to be commercialized in the se
- Opinion
- [Reporter's view] It's time to share the achievements
- by Lee, Seok-Jun Jun 13, 2023 05:43am
- Biopharmaceutical companies attend major overseas conferences in late May and early June. ASCO, EULAR, and BioUSA. During this period, it is said that key workers in the R&D and BD fields are not in Korea. Looking at BIO USA alone, more than 500 Korean companies participated in the event, second only to the US. It is truly a global conferenc
- Company
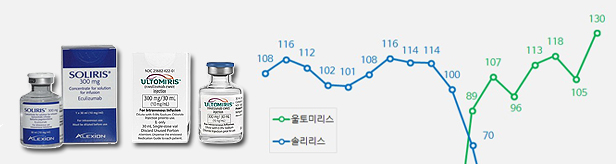
- Sales of Soliris and Ultomiris rise despite changes
- by Kim, Jin-Gu Jun 13, 2023 05:43am
- Quarterly sales of the rare disease treatments ‘Soliris (eculizumab)’ and ‘Ultomiris (ravulizumab)’ had risen significantly in Q1 this year. Although sales of Soliris remained similar to last year, sales of Ultomiris, its follow-on drug, soared in the same period. The analysis is that the generation change between the two drugs has neare
- Company
- Beova can be prescribed at general hospitals
- by Eo, Yun-Ho Jun 12, 2023 05:42am
- Beova, a new drug introduced by Jeil Pharmaceutical, will be available for Rx at general hospitals. According to related industries, Beova, an overactive bladder treatment, is currently available at 22 medical institutions nationwide, including SMC and AMC, as well as advanced general hospitals, Gangnam Severance Hospital, Sangnam Sacred Hear
- Company
- Eisai applies for approval of its AD drug lecanemab in KOR
- by Jung, Sae-Im Jun 12, 2023 05:42am
- The drug that is being considered the next blockbuster candidate, Eisai and Biogen’s Alzheimer’s treatment ‘lecanemab,’ is set to enter the Korean market. Eisai announced on the 8th that it had submitted an application for the marketing authorization of lecanemab to the Ministry of Food and Drug Safety to treat mild cognitive impairm
- Company
- Fidanacogene Elaparvovec designated as an orphan drug
- by Eo, Yun-Ho Jun 12, 2023 05:42am
- Fidanacogene Elaparvovec, a one-shot hemophilia treatment, has been designated an orphan drug. The Ministry of Food and Drug Safety recently announced that it has selected Fidanacogene Elaparvovec, Pfizer's hemophilia B gene therapy, as an orphan drug. Fidanacogene Elaparvovec is a method that combines adenoviral vector (AVV) capsid and highl
- Company
- Chong Kun Dang hastens way into diabetes Tx market
- by Kim, Jin-Gu Jun 12, 2023 05:42am
- Chong Kun Dang is working to accelerate the expansion of its diabetes treatment portfolio. In addition to its own ‘Duvie (lobeglitazone),’ the company received approval for diabetes combination drugs that contain 'Januvia (sitagliptin),’ which it had acquired rights for in Korea. The release of such combination drugs is considered the
- Policy
- When will the innovative generic price system be improved?
- by Lee, Tak-Sun Jun 12, 2023 05:42am
- As announced, the government-pharmaceutical working group for PVA improvement plans started on the 1st with the first meeting and got on track. The industry is complaining of frustration as there is no news about the plan for preferential drug prices for innovative new drugs whose working group has already ended or the reorganization of the g
- Company
- Crystal Genomics supplies 2.6 billion Acelex to Russia
- by Lee, Seok-Jun Jun 9, 2023 05:36am
- Crystal Genomics will supply $2 million of Acelex, an osteoarthritis drug, to Russia. According to the company on the 7th, this order is based on a supply contract with PharmArtis International, a Russian state-run pharmaceutical company. This is the second order quantity. The contractual obligation to purchase is $43.86 million. Crystal Geno