- Company
- Chong Kun Dang hastens way into diabetes Tx market
- by Kim, Jin-Gu Jun 12, 2023 05:42am
- Chong Kun Dang is working to accelerate the expansion of its diabetes treatment portfolio. In addition to its own ‘Duvie (lobeglitazone),’ the company received approval for diabetes combination drugs that contain 'Januvia (sitagliptin),’ which it had acquired rights for in Korea. The release of such combination drugs is considered the
- Policy
- When will the innovative generic price system be improved?
- by Lee, Tak-Sun Jun 12, 2023 05:42am
- As announced, the government-pharmaceutical working group for PVA improvement plans started on the 1st with the first meeting and got on track. The industry is complaining of frustration as there is no news about the plan for preferential drug prices for innovative new drugs whose working group has already ended or the reorganization of the g
- Company
- Crystal Genomics supplies 2.6 billion Acelex to Russia
- by Lee, Seok-Jun Jun 9, 2023 05:36am
- Crystal Genomics will supply $2 million of Acelex, an osteoarthritis drug, to Russia. According to the company on the 7th, this order is based on a supply contract with PharmArtis International, a Russian state-run pharmaceutical company. This is the second order quantity. The contractual obligation to purchase is $43.86 million. Crystal Geno
- Policy
- Adtralza is about to be approved in Korea
- by Lee, Hye-Kyung Jun 9, 2023 05:35am
- Domestic product approval for an adult atopic dermatitis treatment developed by LEO Pharma, a Danish pharmaceutical company, is imminent. According to the pharmaceutical industry on the 8th, the Ministry of Food and Drug Safety completed the safety and efficacy review of 'Adtralza 150mg (tralokinumab)', which Leo Pharma applied for. As the
- Company
- Korea’s new drug reimb rate is below OECD average
- by Eo, Yun-Ho Jun 9, 2023 05:35am
- A survey found that Korea’s new drug reimbursement rate does not amount to the OECD average. The Korean Research-based Pharmaceutical Industry Association (KRPIA, Chairman: Dong-Wook Oh) recently announced Korea’s new drug release status based on its ‘Global Access to New Medicines Report’ According to the report, it takes longer than
- Company
- Non-covalent BTKi pirtobrutinib receives orphan drug desig
- by Eo, Yun-Ho Jun 9, 2023 05:35am
- The BTK inhibitor ‘pirtobrutinib‘ received an orphan drug designation in Korea. The Ministry of Food and Drug Safety recently announced that it had designated Lilly’s Bruton's Tyrosine Kinase (BTK) inhibitor Jaypirca (pirtobrutinib) as an orphan drug. The subject indication is as monotherapy for adult patients with relapsed or refr
- Company
- Humira with sales of 100 billion won
- by Chon, Seung-Hyun Jun 9, 2023 05:35am
- In the autoimmune disease treatment drug ‘Humira’ market, biosimilar products are not showing remarkable activity in the early stages. Although it has been two years since domestic companies penetrated the market, the market share of ‘Humira’ biosimilar has passed two years, but the market share was only 12%. It is analyzed that the price of
- Company
- Daewoong,"promoting to change Fexuprazan's partner"
- by Kim, Jin-Gu Jun 8, 2023 07:47pm
- Daewoong Pharmaceutical is going to change partners in charge of the development and commercialization of Fexuprazan in the North American market. Daewoong Pharmaceutical announced on the 5th that it has terminated the exclusive license agreement with Neurogastrx of the United States for the clinical development and commercialization of Fexup
- Company
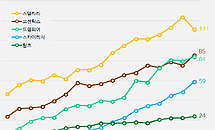
- Competition for interleukin inhibitors heats up in Stelara
- by Kim, Jin-Gu Jun 8, 2023 05:34am
- The interleukin inhibitor market also grew rapidly in the first quarter. Compared to the first quarter of last year, the market size increased by 26% in one year. While Stelara, the market leader, posted sales of KRW 11.1 billion, up 9.8% YoY, generics are rapidly catching up. Skyrizi saw a 69.7% year-over-year increase in sales and Tremf
- Company
- K-Bio appealed for its competitiveness in Bio USA
- by Hwang, Jin-joon Jun 8, 2023 05:33am
- Major domestic pharmaceutical bio companies participated in the '2023 Bio International Convention (Bio USA)', a global partnering event, and introduced their competitiveness. Samsung Biologics disclosed an advanced target operation time for Plant 5 and a plan to build an ADC-only production plant. Lotte Biologics has sought to win biopharma