- Company
- Leading companies in developing new PO microbiome drugs
- by Nho, Byung Chul May 24, 2023 08:28pm
- Recently, the world's first oral microbiome treatment obtained approval from the US Food and Drug Administration (FDA). VOWST from Seres Therapeutics in the U.S. is a drug that prevents re-infection after antibacterial treatment for CDI bacteria for people over 18 years of age. As a new microbiome drug, it is the second after Rebyota of Ferr
- Policy
- Domestic medical device market share ↑50%
- by Lee, Hye-Kyung May 24, 2023 08:26pm
- The share of domestically produced medical devices exceeded 50%. It is believed that the reason is that the production of medical devices such as in vitro diagnostic devices increased as public health medical products were approved for emergency use after Corona 19. Chae Gyu-han, head of the Medical Device Safety Bureau of the Ministry of Food a
- Company
- Pharma industry on alert over business risks in China
- by Lee, Seok-Jun May 24, 2023 05:32am
- Business risks related to China have been rising in Korea’s pharmaceutical industry. There are many causes, including the termination of the contract for supplying medicines (or cosmetics), liquidation of Chinese subsidiaries, and claims for damages, etc. Most of them are due to the failure of their Chinese partners in fulfilling their promises
- Product
- The expansion of OTC medicine at convenience stores
- by Kim JiEun May 24, 2023 05:32am
- As safe household medicines enter their 10th year of introduction, public opinion is forming that the items and places where they can be used should be expanded with the weapon of ‘resolving the gap in medicines and strengthening access rights’. Starting with the application for a special case for demonstration of safe household medicine u
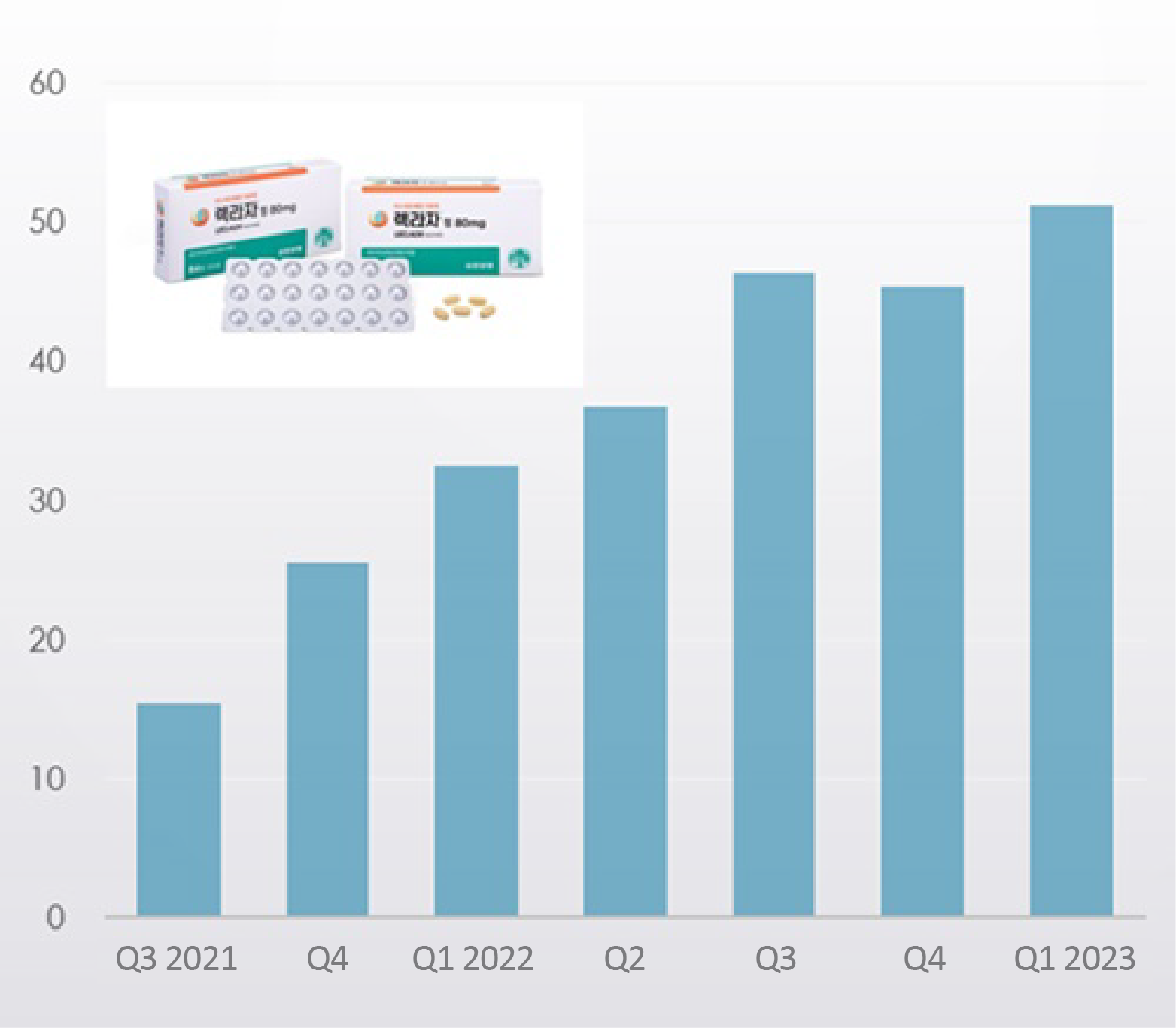
- Company
- Leclaza posts sales of KRW 25 bil in 2 years in Korea
- by Chon, Seung-Hyun May 24, 2023 05:32am
- Yuhan Corp’s anticancer drug ‘Leclaza’ is making good sales in the Korean market, and raised sales of KRW 5.1 billion in Q1 alone. Its efficacy and safety were confirmed in the real world in actual patients at the time of treatment, and the drug is gradually expanding its market influence ahead of its approval as a first-line treatment. Ac
- Company
- Merck retrieves rights to PD-L1 antibody Bavencio in Korea
- by Eo, Yun-Ho May 23, 2023 05:54am
- The long-standing collaboration that had existed between the Korean subsidiaries of Merck and Pfizer Korea for the immunotherapy ‘Bavencio’ has come to a close. According to industry sources, the companies are in the process of handling the rights for the PD-L1-inhibiting immunotherapy Bavencio (avelumab) in Korea as Merck retrieved th
- Company
- RWD results reaffirm Leclaza’s efficacy in practice
- by Chon, Seung-Hyun May 23, 2023 05:54am
- Study results that confirm the efficacy and safety of Yuhan Corp's new anticancer drug ‘Leclaza’ in the real world has been released. Lim Sun Min, Professor of Oncology at Yonsei Cancer Center, and Beung-Chul Ahn, Professor of Oncology at the National Cancer Center, met with reporters at the Korea Pharmaceutical and Bio-Pharma Manufact
- InterView
- The use of HTN combi tx in Korea is the highest in the world
- by May 23, 2023 05:53am
- Korea is the best in the world for complex drugs related to hypertension and hyperlipidemia. The drugs prescribed for each patient’s condition will change, but recently there is a growing trend to use complex drugs that are convenient to take.” Park Chang-gyu, chairman of the Society for Hypertension (Professor, Cardiovascular Center, Kor
- Policy
- Domestic DM combi drugs selected for market competitiveness
- by Lee, Tak-Sun May 23, 2023 05:50am
- LG Chem and Dong-A ST are attracting attention by listing their DPP4i+SGLT2i combination as benefits, lowering the amount added. It is interpreted as being conscious of price competitiveness. According to the industry on the 22nd, Dong-A ST Sugadapa set the upper limit lower than the formula based on addition. This drug is an improved new
- Company
- SGLT2 lowers BP, but more evidence is needed to use it alone
- by Hwang, Jin-joon May 23, 2023 05:50am
- There was an opinion that there is still insufficient evidence for the use of the sodium-glucose cotransporter-2 (SGLT-2) inhibitor, a treatment for type 2 diabetes and heart failure, for the treatment of hypertension. It can be expected to lower blood pressure in patients with heart failure or diabetes, but it is difficult to use it alone f