- Company
- HLB applies for liver cancer drug approval to FDA
- by Lee, Seok-Jun May 19, 2023 05:47am
- HLB submitted a New Drug Application (NDA) to the FDA for Rivoceranib, a targeted anti-cancer drug under development as a first-line liver cancer drug. This is the first time that a domestic bio company has completed its own clinical trials for its anti-cancer drug substance and proceeded with the new drug approval process in the global mark
- Company
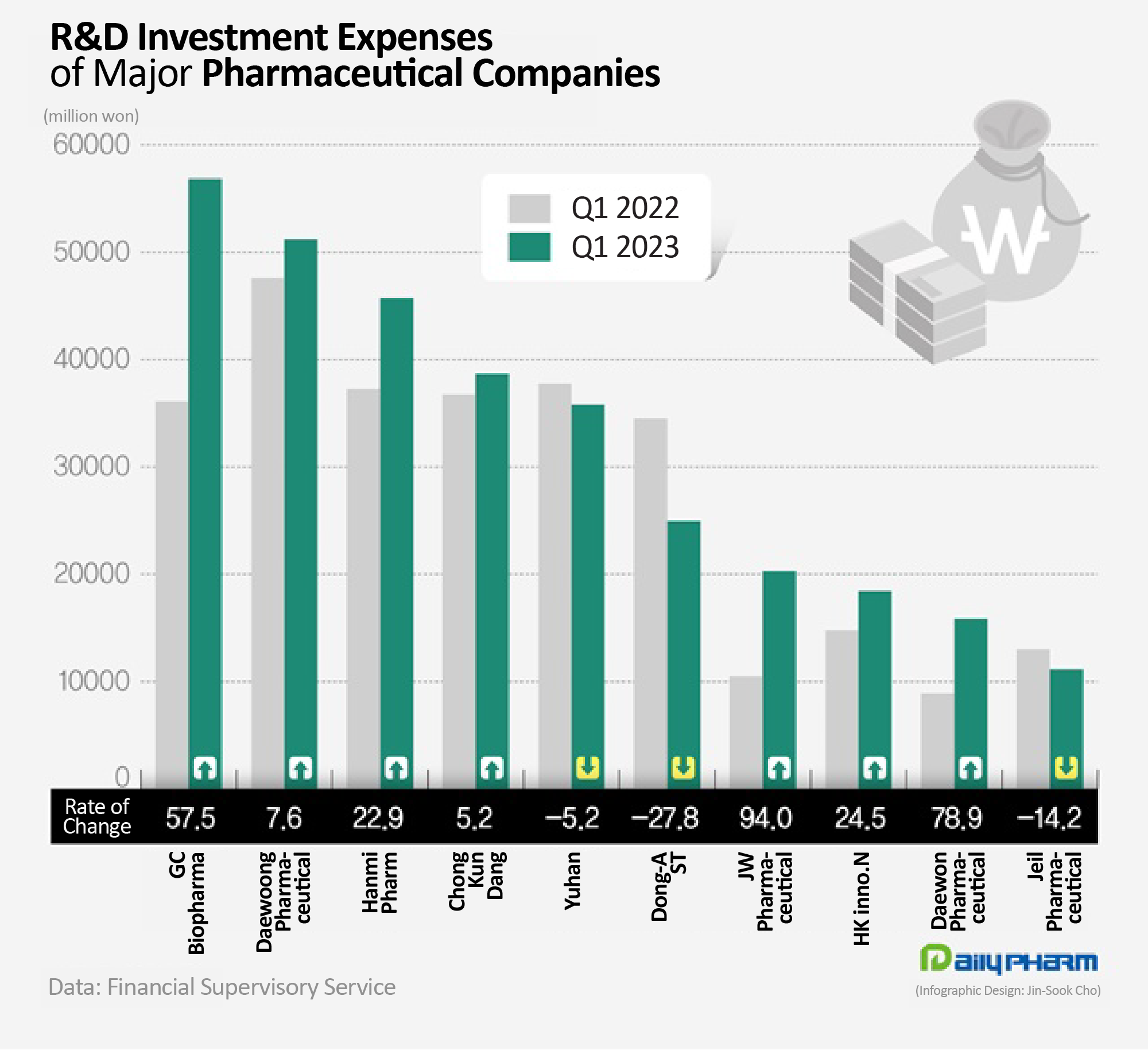
- Korean pharma industry expands R&D investments
- by Chon, Seung-Hyun May 19, 2023 05:47am
- Pharmaceutical companies have vigorously engaged in research and development (R&D) activities to discover future foods. 7 out of 10 major traditional pharmaceutical companies increased their R&D investment compared to last year. R&D expenditures have increased significantly due to the development of new drugs and the introduction of new R&D pipe
- Company
- 90% of pricing managers unsatisfied with new drug price
- by Eo, Yun-Ho May 19, 2023 05:46am
- Study results showed that about 90% of the drug pricing managers in Korea are not satisfied with the value recognized for new drugs. Recently, a study on ‘'An Industry Survey on Unmet Needs in South Korea’s New Drug Listing System' was published on the online version of the medical science journal Springer (https://link.springer.com/art
- Company
- Ibrance emerges as a new drug partner for metastatic breast
- by Jung, Sae-Im May 19, 2023 05:46am
- CDK4/6 inhibitor Ibrance is emerging as a combination partner for metastatic breast cancer drug developers based on its long-accumulated treatment experience. Even if the dose is increased, there is little concern about side effects, so it is expected that it will be used as a variety of combination drugs. Ibrance is the first CDK4/6 inhibit
- Policy
- Revival of omega-3 fatty acids...4g high-dose recommended
- by Choi, sun May 18, 2023 05:45am
- The Korean Society of Lipid and Atherosclerosis (KSoLA) disclosed the full version of its 5th edition of the Korean Guidelines for the Management of Dyslipidemia, in which the use of omega 3 was subdivided into the use of 'high dose and refined ingredients'. Although there has been controversy over its efficacy, the new guideline puts weight on
- Product
- Diabetes Association also paid attention to 'zero' drinks
- by Choi, sun May 18, 2023 05:45am
- Possible inhibition of glycemic response and increased risk of cardiovascular events This year, while the American Diabetes Association recognized intermittent fasting and time-restricted eating as part of a meal pattern based on research results that help reduce weight and improve blood sugar, the Korean Diabetes Association also reviewed low-c
- Policy
- Initial appvl rate of drugs subject to prior review varies
- by Lee, Tak-Sun May 18, 2023 05:45am
- As a result of analyzing the approval rate of prior authorization drugs over the past 10 years, the approval rate varied greatly according to the type of drug. However, unlike during the initial review, the review for maintenance therapy showed a high approval rate of 90%. Yong-Kyun Won, Professor of Radiation Oncology at Soonchunhyang Uni
- Opinion
- [Reporter's view] Are you ready to use
- by Lee, Hye-Kyung May 18, 2023 05:45am
- The 'Act on the Safety and Support of Advanced Recycles and Advanced Biomedicines' will be in effect for three years in August. The Advanced Recycled Bio Act prepares a system for securing the safety of advanced renewable medicine, provides a plan for technological innovation, and practical use, and stipulates the necessary matters to secure
- Company
- Chong Kun Dang has the domestic license for the 110 billion
- by Chon, Seung-Hyun May 18, 2023 05:44am
- Chong Kun Dang bought the domestic license for MSD’s blockbuster diabetes treatment ‘Januvia series’. It is equipped with a stable cash cow that raises more than 100 billion won a year. Chong Kun Dang signed a license agreement with MSD headquarters in Switzerland to introduce all domestic rights for three diabetes treatments, Januvia, Janume
- Company
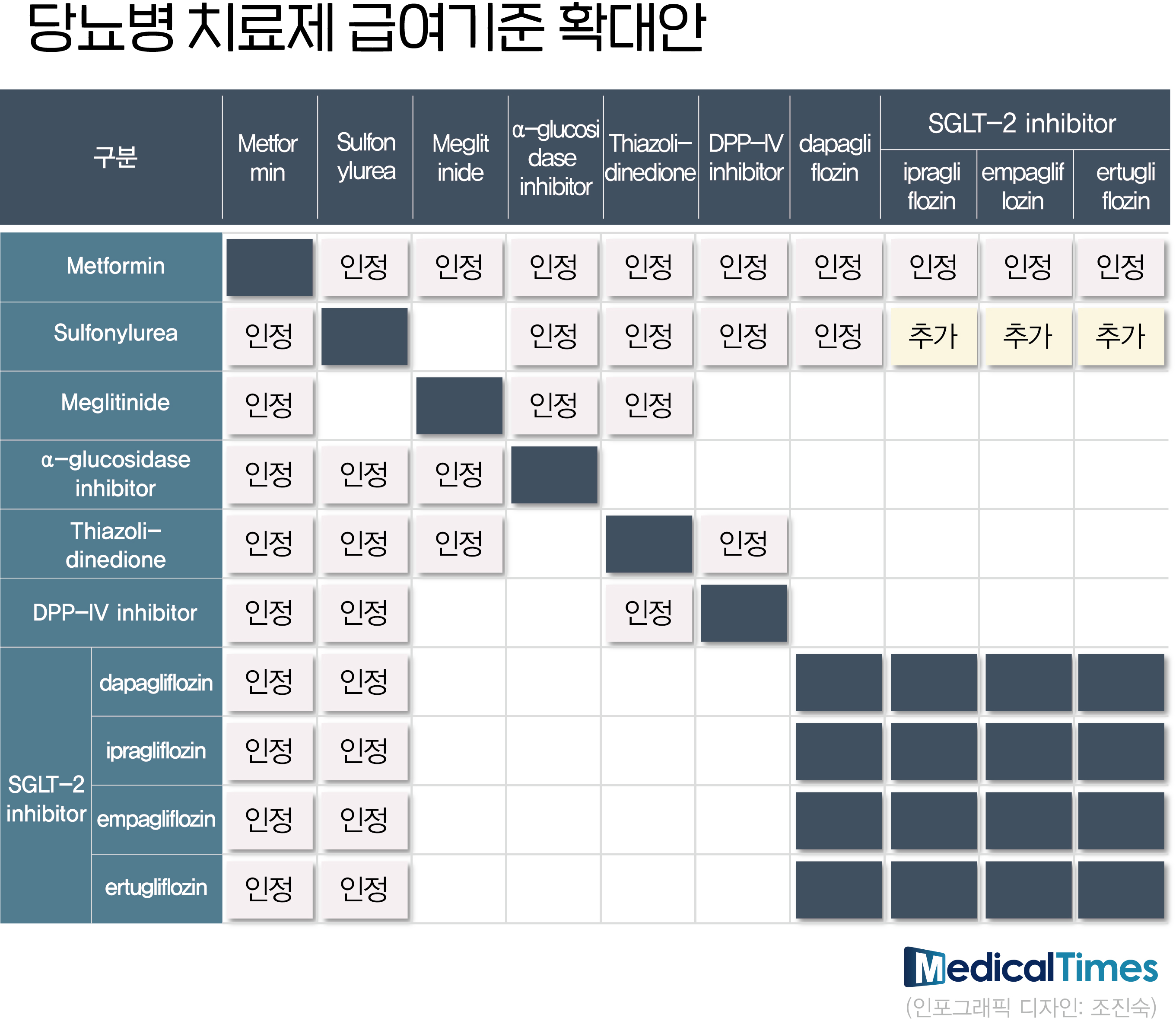
- Benefit extended DM Drug
- by Moon, sung-ho May 17, 2023 05:38am
- Clinical sites are busy finding the optimal prescription combination while the expansion of diabetes treatment reimbursement standards for each class and the release of generics following the patent expiration of original items coincided. It is an effort to find the optimal combination for each treatment category that can be covered by health