- Company
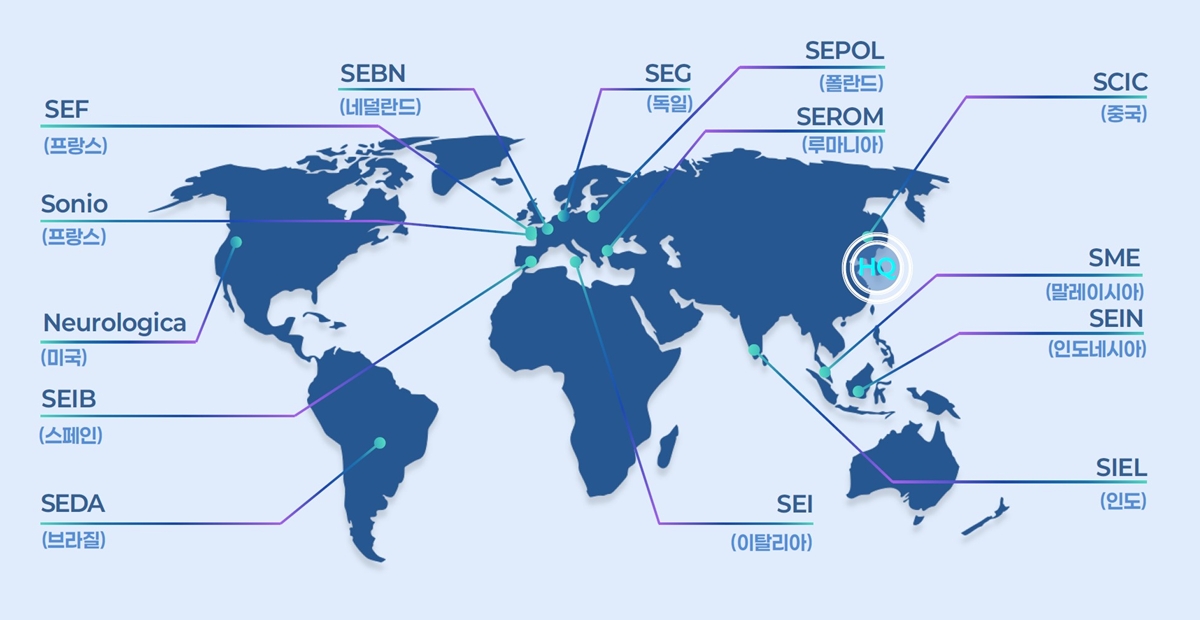
- Samsung Medison aims to achieve 'competitive edge in tech'
- by Whang, byung-woo Jul 22, 2025 06:09am
- Samsung Medison, a leading company in South Korea's medical device industry, is celebrating its 40th anniversary this year by investing heavily to become a top player in the diagnostic ultrasound equipment sector. Over the past 40 years, Samsung Medison has grown into a global medical device company, supplying diagnostic ultrasound equipme
- Company
- Astellas Korea's Seonhee Lee appointed GM of Astellas Egypt
- by Whang, byung-woo Jul 22, 2025 06:07am
- Astellas Korea announced on July 21 that the company has appointed Seonhee Lee, currently a Commercial Excellence & Market Access Head at Astellas Pharma, as the new General Manager of Astellas Egypt, effective from August 1.. Lee has 23 years of experience working in major pharmaceutical companies in South Korea and overseas and is a he
- Company
- Original Prolia will remain ultimate despite patent expiry
- by Whang, byung-woo Jul 22, 2025 06:07am
- As an innovative new drug that transformed the treatment paradigm for osteoporosis, Prolia reached its peak last year with record-high sales. However, with its patent expiration in March this year and the looming entry of biosimilars, the market landscape is expected to undergo significant changes. Amgen Korea plans to solidify Prolia's p
- Company
- Merck Life Science-KAIST hold workshop to share achievements
- by Whang, byung-woo Jul 22, 2025 06:06am
- Merck Life Science Korea announced on the 21st that it held the “Merck-KAIST Workshop” in Boston, USA, for two days from July 17th to 18th in collaboration with KAIST. The workshop marked the first anniversary of the strategic partnership signed between Merck Life Science and KAIST in May last year, and served as a venue to share achiev
- Company
- Minimally invasive 'da Vinci robotic surgery'
- by Whang, byung-woo Jul 22, 2025 06:05am
- As robotic surgery becomes a global standard, the industrial robotics market continues to grow in size. In South Korea, where Intuitive Surgical's first Asian branch was established, the market presence of da Vinci robotic surgery is increasingly broadening as it marks its 20th anniversary since its introduction to the country. Intuitive S
- Company
- LEO Pharma’s 117-year, single-focus strategy
- by Cha, Jihyun Jul 21, 2025 06:08am
- LEO Pharma is a representative symbol of the success of the Danish biotech industry. It is the oldest pharmaceutical company in Denmark, celebrating its 117th anniversary this year. Originally founded as a pharmacy in Copenhagen, Denmark, the company has grown based on its philosophy of standardizing the quality of medicines and supplying
- Company
- Omjjara may be prescribed at general hospitals in KOR
- by Eo, Yun-Ho Jul 21, 2025 06:07am
- The new myelofibrosis drug ‘Omjjara’ can now be prescribed in general hospitals in Korea. According to industry sources, GSK Korea's myelofibrosis treatment Omjjara (momelotinib) recently passed review by Drug Committees (DCs) of 13 medical institutions, including Samsung Medical Center, Seoul St. Mary's Hospital, Asan Medical Center Se
- Policy
- Jardiance 10mg price reduced from ₩618 to ₩582
- by Lee, Tak-Sun Jul 21, 2025 06:06am
- The maximum insurance price ceiling for the 10 mg flagship dosage form of the SGLT-2 inhibitor diabetes treatment Jardiance Tab (empagliflozin, Boehringer Ingelheim), will be adjusted as reimbursement will also apply to chronic kidney disease.&160; Jardiance recorded KRW 66.3 billion in outpatient prescriptions (based on UBIST) last year
- Company
- Immunotherapy 'Jemperli' enters drug price negotiations
- by Eo, Yun-Ho Jul 21, 2025 06:06am
- Jemperli, an immunotherapy for cancer, has entered the last stage for its expanded reimbursement for endometrial cancer. According to industry sources, GSK Korea has initiated the drug price negotiations with the National Health Insurance Service (NHIS) for its PD-1 inhibitor, Jemperli (dostarlimab). The detailed expanded reimbursement
- Policy
- [Reporter's View] A separate fund for new orphan drugs
- by Lee, Jeong-Hwan Jul 18, 2025 06:36am
- Will the 'establishment of a separate fund,' considered one of the ways to improve patient access to expensive, rare, and intractable disease drugs, become even more difficult with the inauguration of the Lee Jae Myung administration? Jung Eun-kyung, the candidate for Minister of Health and Welfare (MOHW), who is facing a parliamentary co