- Policy
- Started a demand survey for drugs participating in the pilot
- by Lee, Tak-Sun Mar 31, 2023 06:08am
- Due to the burden of simultaneous data submission, there are likely to be fewer participating drugs. The government started a demand survey for drugs to participate in the parallel trial project of 'Permission Evaluation Negotiation', which is being pursued rapid reimbursement. The project was planned to drastically shorten the registration
- Policy
- About 180 clinics without IRB used diagnostic drugs
- by Park,Yang-myeong Mar 30, 2023 05:42am
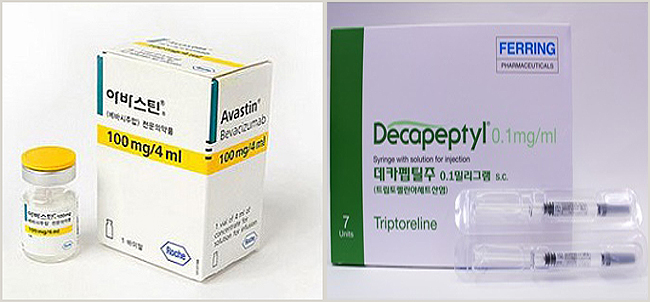
- Avastin and Decapeptyl, are drugs that allow the use of drugs beyond the scope of approval without the IRB. How much is it used in front-line medical institutions? About 230 macular degeneration treatment drugs Avastin and precocious puberty drug Decapeptyl injection were found to be using these drugs without IRB at 180 hospitals and clinics.
- Company
- Viatrice Korea appoints Bill Schuster as new CEO
- by Jung, Sae-Im Mar 30, 2023 05:42am
- Viatris Korea announced on the 28th that it would appoint Bill Schuster as its new CEO. New CEO Bill Schuster is an expert with more than 30 years of diverse business experience in the healthcare and pharmaceutical industries. Based on his career as a senior leader in Asia, the Americas, and Europe, he is highly regarded for his understand
- Policy
- New Pompe disease drug Nexviazyme is approved in Korea
- by Lee, Hye-Kyung Mar 30, 2023 05:41am
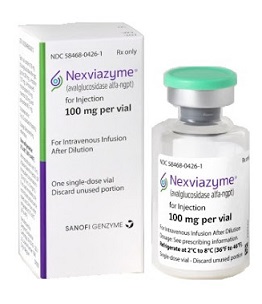
- The long-term enzyme replacement therapy for Pompe disease, Nexviazyme Inj (avalglucosidase alfa-ngpt), has received marketing authorization in Korea. On the 29th, the Ministry of Food and Drug Safety (Minister Yu-Kyung Oh) announced that it had approved Sanofi-Aventis Korea’s Nexviazyme, a treatment for a rare condition called Pompe dis
- Opinion
- [Reporter’s View] Accepting drugs used by very few patients
- by Eo, Yun-Ho Mar 30, 2023 05:41am
- It's the same 'cancer', but different. So how should we regard these new anticancer drugs that target only a very few among all patients that have the same type of cancer? The cancer types that we commonly refer to, such as liver cancer, stomach cancer, and lung cancer are just major categories used, and the specific condition of each pat
- Policy
- Obizur will be evaluated for reimbursement upon approval
- by Lee, Tak-Sun Mar 30, 2023 05:41am
- Obizur, a treatment for acquired hemophilia A for adults, approved on the 20th, immediately began a benefit evaluation. This drug is attracting attention as it is the first treatment to replace blood coagulation factor 8 with an indication for acquired hemophilia A. According to the industry on the 29th, the HIRA received an application fo
- Policy
- Govt seeks to improve the drug price reduction system
- by Lee, Tak-Sun Mar 29, 2023 06:02am
- The Korean government will be working together with the pharmaceutical industry to come up with plans to improve the price reduction system that discounts the actual transaction price of drugs. The Health Insurance Review and Assessment Service plans to hold a roundtable meeting with the pharmaceutical industry to improve the actual dru
- Company
- JW Pharma registers the first overseas patent for JW0061
- by Mar 29, 2023 06:02am
- JW Pharmaceutical announced on the 27th that it has obtained a patent for 'JW0061', a treatment for hair loss targeting Wnt, from the Russian Intellectual Property Office. This patent is for JW0061, a new drug candidate for hair loss treatment based on the Wnt signaling pathway. The patent protects the composition of JW0061. This is the fi
- Company
- Bridge Biotherapeutics applies to FDA for the phase 1/2 plan
- by Mar 29, 2023 06:02am
- Bridge Biotherapeutics announced on the 27th that it had applied to the FDA for a phase 1/2 clinical trial plan for 'BBT-207', a 4th generation non-small cell lung cancer drug candidate. BBT207 is a new drug candidate that targets the C797S mutation that can occur after using third-generation non-small cell lung cancer treatments such as Tagr
- Company
- TB treatment Dovprela lands in general hospitals in Korea
- by Eo, Yun-Ho Mar 29, 2023 06:02am
- ‘Dovprela,’ the first new drug introduced in the field of tuberculosis in half a century can now be prescribed at general hospitals in Korea. Viatris Korea's multidrug-resistant tuberculosis treatment Dobprela (pretomanid) passed the Drug Committees (DCs) of Seoul National University Hospital and Seoul Asan Medical Center. Dovprela,