- Policy
- Dupixent 200mg, newly listed
- by Kim, Jung-Ju Mar 27, 2023 05:55am
- Sanofi-Aventis Korea's severe atopic dermatitis treatment Dupixent PFS 300mg has expanded insurance coverage to pediatric and adolescent patients, and products with 200mg content will be newly listed next month with an expanded range. Taking this as an opportunity, the government is planning to expand the scope of benefits for special calculatio
- Policy
- Forxiga patent is about to expire
- by Kim, Jung-Ju Mar 24, 2023 05:48am
- As the patent for Dapagliflozin, the original drug (Forxiga), is about to expire, domestic generics of this drug will be released early next month. Accordingly, the government is swiftly proceeding with the process of calculating the drug price and additional negotiations so that the drug can be listed immediately. According to the industr
- Policy
- Dual punishment for CSO rebates passes NA committee
- by Lee, Jeong-Hwan Mar 24, 2023 05:48am
- A provision that stipulates the prohibition of doctors from receiving rebates offered by contract sales organizations (CSOs) and a plan to amend the Medical Service Act to advance regulations on medical advertisements and allow the Ministry of Health and Welfare to intervene in the Medical Advertisement Deliberation Committee passed the plen
- Company
- Discussion of reimbursement for Vyndamax is running in place
- by Eo, Yun-Ho Mar 24, 2023 05:48am
- It seems that discussions on registration of transthyretin (TTR) amyloid cardiomyopathy drug 'Vindamax' for insurance benefits are at a standstill again. As a result of the coverage, Pfizer Korea's ATTR-CM (ATTR amyloidosis with cardiomyopathy) treatment Vyndamax, (Tafamidis 61mg) passed the HIRA last year, but the schedule for presentatio
- Company
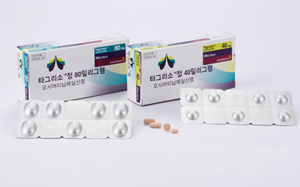
- 5th time the charm for Tagrisso’s reimbursement extension?
- by Jung, Sae-Im Mar 24, 2023 05:48am
- After five attempts, the EGFR mutation-positive non-small cell lung cancer (NSCLC) treatment ‘Tagrisso (osimertinib)’ finally crossed the first hurdle to receiving reimbursement as a first-line treatment. Although other procedures such as deliberation by the Health Insurance Review and Assessment Service’s Drug Reimbursement Evaluation Co
- Policy
- Rinvoq also succeeded in expanding youth atopy benefits
- by Lee, Tak-Sun Mar 24, 2023 05:48am
- Rinvoq 15mg will be covered from April as an adolescent atopy treatment. On the same day as the biologic drug Dupixent, the youth atopic treatment benefit is applied. It is the first among JAK inhibitors to obtain reimbursement for the youth indication before Cibinqo, a competitor drug. According to the industry on the 22nd, Rinvoq SR 15mg wi
- Opinion
- [Reporter's view]Vaccine self-sufficiency rate to 80%
- by Kim, Jin-Gu Mar 23, 2023 04:45am
- In 2013, 10 years ago, the Ministry of Health and Welfare distributed a press release. The plan is to foster the domestic vaccine industry and raise the self-sufficiency rate of the National Immunization Vaccine to 80% by 2020. At the time, among the 28 types of National Immunization Vaccines, only 8 types of vaccines were self-sufficient, b
- Policy
- Pharma industry makes a request to MOTIE and MOEF
- by Nho, Byung Chul Mar 23, 2023 04:45am
- Public opinion has been rising for the government to revoke the designation of botulinum toxin as a national core technology. The rise of this public opinion brings more meaning as the request was made by Korea Pharmaceutical and Bio-Pharma Manufacturers Association, which represents the industry. Breaking away from lesser movements that
- Policy
- Legislation requiring submission of botulinum strains
- by Lee, Jeong-Hwan Mar 23, 2023 04:45am
- A bill that makes it mandatory to submit genetic information of bioterrorism infectious diseases such as botulinum toxin to domestic quarantine authorities and cancels the possession permit if not submitted did not pass the Health and Welfare Committee of the National Assembly held on the 22nd. If the bill is passed, it may lead to excessive
- Company
- Neurofibromatosis Tx Koselugo makes progress for reimb
- by Eo, Yun-Ho Mar 23, 2023 04:45am
- Finally, reimbursement discussions for the new neurofibromatosis drug ‘Koselugo’ has made some progress. Results showed that AstraZeneca Korea’s new neurofibromatosis drug has passed the review by the Health Insurance Review and Assessment Service’s Drug Reimbursement Standard Subcommittee recently. Therefore, its reimbursement can no