- Policy
- Pfizer’s Cibinqo to undergo NHIS negotiations for reimb
- by Lee, Tak-Sun Mar 13, 2023 05:53am
- Pfizer has accepted the conditions set by the Health Insurance Review and Assessment Service’s Drug Reimbursement Evaluation Committee for the reimbursement of its Cibinqo Tab (abrocitinib) in Korea. The drug received conditional approval at the DREC meeting that was held on March 2. The committee deemed Cibinqo’s reimbursement will be
- Company
- GC Pharma begins develop of mRNA flu vaccine candidates
- by Hwang, Jin-joon Mar 10, 2023 05:50am
- Invested in mRNA pilot production facilities in Hwasun Vaccine Plant in Jeollanam-do. GC Pharma announced on the 9th that it will apply Acuitas Therapeutics' Lipid Nano Particle (LNP) technology to develop mRNA flu vaccine candidates in earnest. GC Pharma signed an LNP-related Development and Option Agreement with Acuitas in Canada in April o
- Policy
- Industry unsatisfied with the proposed PVA improvements
- by Lee, Tak-Sun Mar 10, 2023 05:50am
- Companies that have released new drugs in Korea have expressed discontent over the measures to improve the price-volume agreement (PVA) system that had been recently disclosed by the National Health Insurance Service. The new measure will put drugs that undergo PVA Type A negotiations at a relative disadvantage. According to the measur
- Company
- Myelofibrosis New Drug Inrebic
- by Eo, Yun-Ho Mar 10, 2023 05:50am
- Inrebic, a myelofibrosis treatment option born 10 years after Jakavi, is accelerating its steps toward insurance coverage. As a result of the coverage, BMS Pharmaceutical's myelofibrosis treatment Inrebic is in the process of drug price negotiations with the NHIS. Depending on the negotiation date, it is expected that it will be possible to
- Company
- Enbrel's share is 44% and Herceptin's share is 37%
- by Kim, Jin-Gu Mar 10, 2023 05:50am
- Mabthera, Avastin, and Humira similars also saw a sharp rise in market share new product addition effect. The share of biosimilar products in the domestic market is rapidly expanding. Enbrel biosimilars Etanercept increased its market share from 12% in 2018 to 44% last year. Herceptin similars also expanded from 9% to 37% during the same per
- Policy
- Dupixent, Cibinqo, Rinvoq to be reimb for pediatric AD in 1H
- by Lee, Tak-Sun Mar 10, 2023 05:49am
- The three drugs that are attempting to receive reimbursement as a treatment for pediatric·adolescent patients with atopic dermatitis - Dupixent (dupilumab), Cibinqo (abrocitinib), and Rinvoq (upadacitinib) &8211; are expected to be approved for reimbursement within the first half of the year. With all 3 drugs passing the Health Insuran
- Company
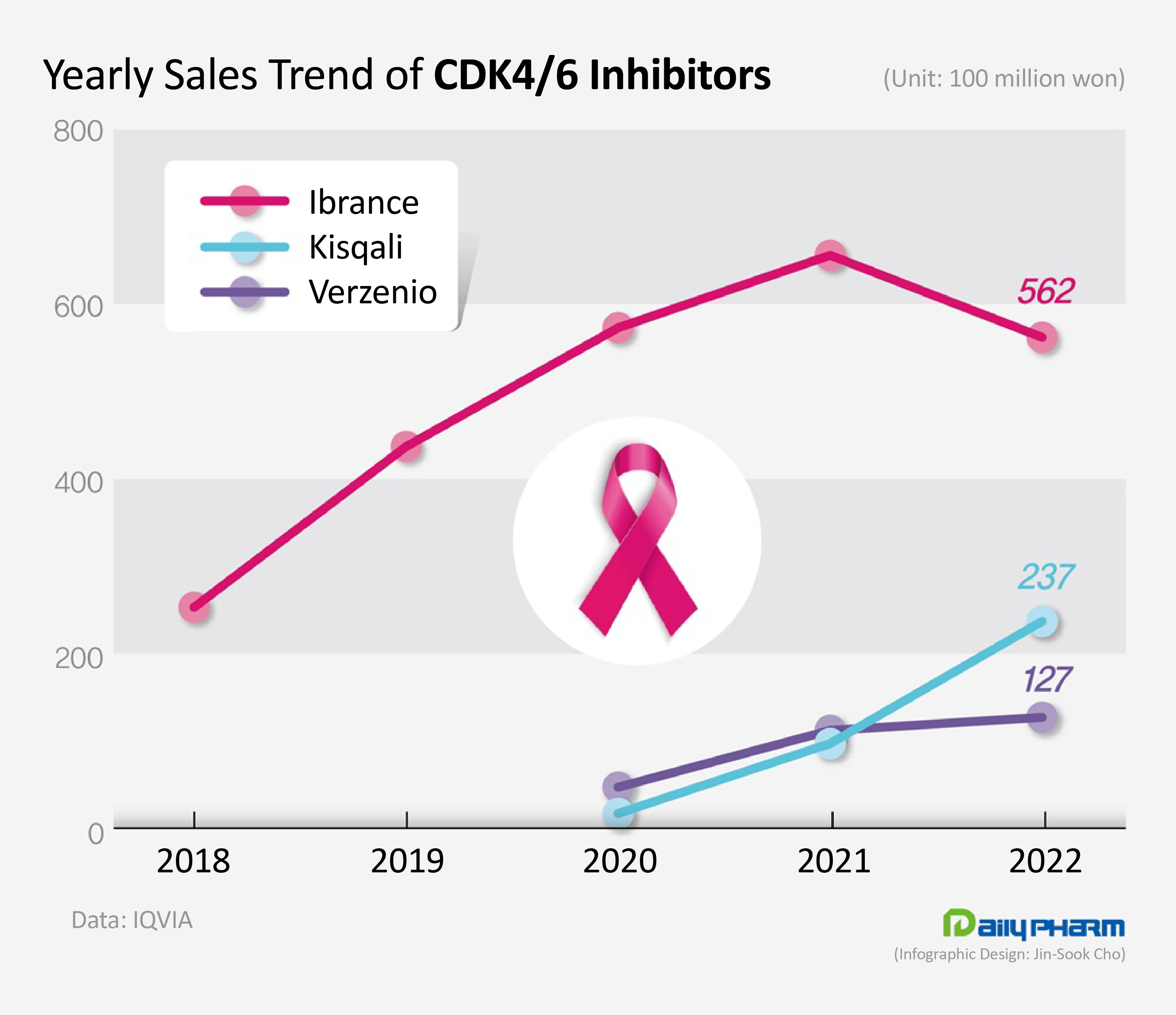
- Sales of Kisqali surge, Ibrance decline
- by Jung, Sae-Im Mar 9, 2023 06:00am
- The market for cyclin-dependent kinases (CDK) 4/6 inhibitors that are used to treat metastatic breast cancer have undergone drastic changes last year. Sales of Ibrance, which used to dominate the market, had faltered, while the latecomer Kisqali rapidly expanded its share in the market . According to the market research institution IQVIA
- Policy
- Multiple myeloma CAR-T therapeutic agent CARVYKTI, imminent
- by Lee, Hye-Kyung Mar 9, 2023 06:00am
- CARVYKTI compares self -hematopoietic stem cell transplantation (ASCT) after administration of Daratumumab, Bortezomib, Lenalidomide, and DVRD in December last year. The clinical trial was conducted. According to the industry on the 8th, the Ministry of Food and Drug Safety recently completed the safety and validation of Carvykti. If the Effi
- Company
- Childhood dementia Tx Xenpozyme to soon land in KOR
- by Eo, Yun-Ho Mar 9, 2023 06:00am
- The first childhood dementia treatment is expected to be commercialized in Korea soon. According to industry sources, the Ministry of Food and Drug Safety is conducting the final review to approve Sanofi Genzyme’s treatment for acid sphingomyelinase deficiency (ASMD)m ‘Xenpozyme (olipudase alfa).’ Starting with Japan in March, the d
- Company
- Hemlibra is also effective for mild and secondary hemophilia
- by Kim, Jin-Gu Mar 9, 2023 06:00am
- JW Pharma announced on the 6th that Phase III clinical trials that proved the effects and safety of patients with type hemophilia have been published in the online edition of the Lancet Hematology 2023, an international journal. Hemlibra is a type A hemophilia disease caused by the deficiency of factor XIII. It is the only anti-antibody pat