- Company
- How Denmark gave birth to the golden goose Wegovy
- by Cha, Jihyun Jul 17, 2025 06:13am
- Novo Nordisk, the Danish company that developed the obesity treatment Wegovy that shook the world, rose to the top in Europe in terms of market capitalization in 2023. It surpassed France's luxury goods group LVMH, which had held the top spot in the European stock market for over two years. At the time, Novo Nordisk’s market value was appr
- Company
- Oral lung cancer drug market race
- by Son, Hyung Min Jul 16, 2025 06:10am
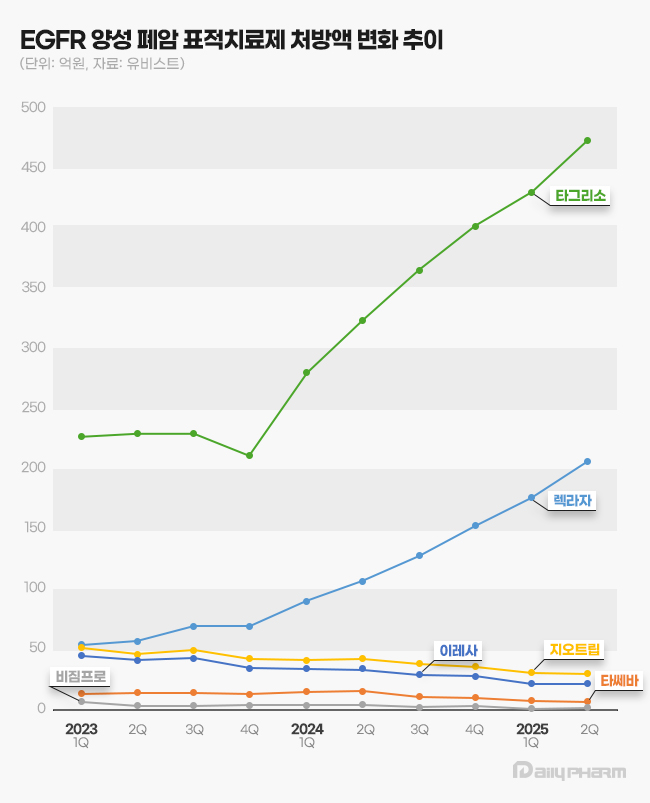
- Late entrants, such as Leclaza and Lorviqua, are expanding their presence in the Korean lung cancer targeted therapy market, continuing rapid growth. While prescription sales for some EGFR and ALK-positive non-small cell lung cancer (NSCLC) treatments are stagnating or declining, newer drugs in these categories are showing a clear upward trend,
- Opinion
- [Reporter's View] Gov’t cooperation leads to a price cut?
- by Eo, Yun-Ho Jul 16, 2025 06:10am
- There are times when cooperation ends up causing losses. In an ironic twist, pharmaceutical companies that participated in the government's infertility support program have now found their products caught in the net of the Price-Volume Agreement (PVA) system, leaving them little choice but to face rapid drug price cuts. The government's i
- Company
- What benefit will BeOne Medicines’ Tevimbra bring?
- by Whang, byung-woo Jul 16, 2025 06:10am
- Tevimbra (tislelizumab), the first immune-oncology drug to be reimbursed for the treatment of esophageal cancer, is expanding its indications to penetrate the market. In a market already dominated by established immune-oncology drugs such as Keytruda (pembrolizumab) and Opdivo (nivolumab), pricing and scalability are expected to be key strate
- Company
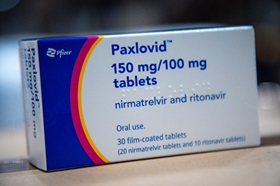
- Paxlovid prescriptions exceed ₩10B in Q2 amid resurge
- by Kim, Jin-Gu Jul 16, 2025 06:09am
- Quarterly prescriptions for the COVID-19 treatment Paxlovid have surpassed KRW 100 billion. Since entering the prescription market in October last year, usage has rapidly increased, with cumulative prescriptions reaching KRW 23.7 billion. This is believed to be due to the resurgence of COVID-19 in South Korea in March and April this year.
- Policy
- Will the general diabetes criteria be revised after 14 yrs?
- by Lee, Tak-Sun Jul 16, 2025 06:09am
- The National Health Insurance Service (NHIS) is currently reviewing a comprehensive revision of the general principles for diabetes drug reimbursement criteria, which have been in place for 14 years. It is garnering significant attention from the industry. Following recent trends, significant changes are anticipated, including the remova
- Company
- Potential changes to reimb policy for new anticancer drugs
- by Moon, sung-ho Jul 16, 2025 06:08am
- Multinational pharmaceutical companies are actively pursuing to secure reimbursement for their anti-cancer drugs as first-line treatment options. Based on accumulated clinical research, companies are competitively applying for reimbursement from the government, putting in all efforts to accomplish this by the second half of this year. R
- Company
- Polivy’s reimbursement for DLBLC will soon be reviewed
- by Eo, Yun-Ho Jul 15, 2025 06:08am
- Polivy, the first first-line treatment introduced for diffuse large B-cell lymphoma (DLBCL) in 20 years, is entering the first stage of its renewed bid for reimbursement in Korea. According to industry sources, Polivy (polatuzumab vedotin), a treatment developed by Roche Korea for relapsed or refractory diffuse large B-cell lymphoma (DLBC
- Policy
- Gov't pursues 'project to produce unstable supply drugs'
- by Lee, Jeong-Hwan Jul 15, 2025 06:08am
- The Ministry of Health and Welfare (MOHW) plans to continue its project of providing government funding for the production of drugs with unstable supply. The MOHW plans to proceed with the currently budgeted project to support one item, while also working to secure additional budget to support more items. A MOHW official recently met w
- Opinion
- [Reporter’s View]Biotech’s responsible approach to failure
- by Cha, Jihyun Jul 15, 2025 06:06am
- Cases of terminated licensing deals and halted clinical trials are on the rise in Korea's biotech sector. Recently, IntoCell announced that the licensing agreement for its ADC (antibody-drug conjugate) platform with ABL Bio had been canceled due to patent overlap issues. Prior to that, Orum Therapeutics voluntarily decided to halt clinical d