- Company
- Various immunotherapies attempt to treat Alzheimer’s
- by Son, Hyung Min Jul 15, 2025 06:06am
- A series of immunotherapy candidates are showing promise in the field of Alzheimer's disease treatment. With the limitations of existing antibody therapies becoming apparent, multinational pharmaceutical companies are attempting approaches targeting new immune pathways. In particular, pipelines targeting innate immune regulation, such as NK cell
- Company
- Omjjara’s reimbursement progress gains attention
- by Eo, Yun-Ho Jul 14, 2025 06:04am
- Attention is turning to whether progress will be made in the reimbursement listing process for the new myelofibrosis drug, Omjjara. Omjjara (momelotinib), a myelofibrosis treatment developed by GSK Korea, passed the Health Insurance Review and Assessment Service's Cancer Disease Review Committee in March and is currently awaiting submissi
- Company
- Aftermath of Januvia's price cut…MSD-CKD compensation fight
- by Son, Hyung Min Jul 14, 2025 06:04am
- Regarding transaction for price difference compensation following the drug price reduction for the diabetes treatment Januvia, Chong Kun Dang and MSD Korea have not reached agreement during negotiation for nearly two years. While the distribution industry continues to demand compensation for losses incurred due to the drug price cut, the lac
- Policy
- How Trodelvy was applied flexible ICER for innovativeness
- by Lee, Tak-Sun Jul 14, 2025 06:04am
- The ADC breast cancer drug Trodelvy (sacituzumab govitecan, Gilead), which was reimbursed in June for triple-negative breast cancer, was the first case in which the ICER (incremental cost-effectiveness ratio) threshold was flexibly applied in recognition of its innovation. So how did the Drug Reimbursement Evaluation Committee (DREC) of t
- Company
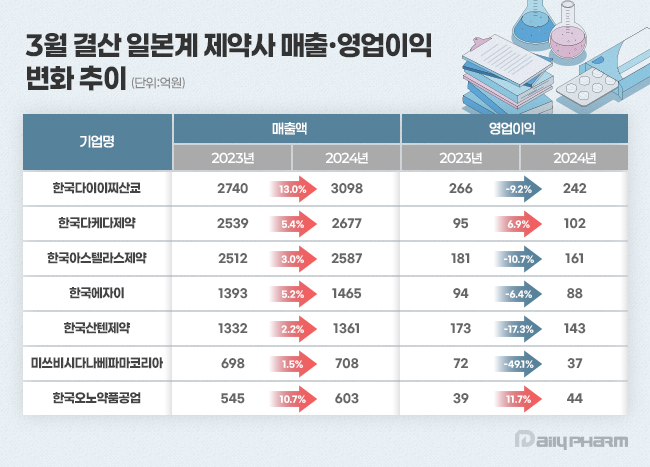
- Daiichi Sankyo and Ono Pharmaceutical see surge in sales
- by Son, Hyung Min Jul 14, 2025 06:03am
- Japanese pharmaceutical companies operating in South Korea saw overall sales growth last year. All seven companies surveyed achieved year-on-year sales growth, with Daiichi Sankyo Korea and Ono Pharma Korea leading the way with double-digit growth rates. However, in terms of operating profits, the companies showed mixed emotions. According to
- Policy
- Orphan drug 'Bylvay Cap' wins reimb approval after reeval
- by Lee, Tak-Sun Jul 14, 2025 06:03am
- 'Bylvay Cap,' which is being considered for the expedited reimbursement listing process as a designated drug for 'Pilot Project for Integration of Product Approvals, Reimbursement Coverage Reviews, and Drug Price Negotiations,' received reimbursement appropriateness decision after reevaluation. Consequently, Bylvay Cap will be subjected to ne
- Company
- Bimzelx may be prescribed at general hospitals in KOR
- by Eo, Yun-Ho Jul 11, 2025 06:12am
- The new psoriasis drug Bimzelx may be prescribed at general hospitals in Korea. According to industry sources, UCB Pharma Korea's Bimzelx (bimekizumab) has been approved by the Drug Committees (DCs) of major hospitals nationwide, including tertiary hospitals like Seoul Asan Medical Center and Severance Hospital as well as major hospitals
- Opinion
- [Reporter's View] US tariffs…variable that must be faced
- by Whang, byung-woo Jul 11, 2025 06:12am
- Tension is growing in the domestic pharmaceutical and biotech industry as President Donald Trump recently announced the potential imposition of tariffs on imported pharmaceuticals. If the tariff hike is imposed, it is expected to directly impact not only the procurement costs of raw materials and sub-materials for Korean companies but als
- Policy
- Will drugs subject to reimbursement reevals be expanded?
- by Lee, Tak-Sun Jul 11, 2025 06:11am
- The government is reportedly planning to tighten the selection criteria ahead of the second phase of the drug reimbursement adequacy reevaluations, which is set to begin next year. As a result, it is expected that the number of ingredients subject to reassessment may be expanded. According to industry sources on the 10th, the Health In
- Policy
- Pfizer discontinues the supply of 'Viviant' in KOR
- by Lee, Tak-Sun Jul 11, 2025 06:09am
- Pfizer announced of discontinuing the supply of its 'Viviant Tab (bazedoxifene acetate)' in Korea. They stated that supply discontinuation is due to the difficulty in securing a stable and continuous supply. Viviant had previously been out of stock in May due to delays in the manufacturing site. An analysis suggests that Viviant's marke