- Company
- The dismissal of 18 employees of Zuellig Pharma is unfair
- by Jan 1, 2023 10:43pm
- According to the pharmaceutical industry on the 29th, the 11th Civil Affairs Department (chief judge Park Tae-il) of the Seoul Western District Court ruled in favor of some of the plaintiffs in a lawsuit filed by 18 fired Zuellig Pharma SSK against the company on the 22nd. According to the Democratic Pharmaceutical Union, the first trial cour
- Company
- Pharmaceutical shares capitalization of ₩56 trillion
- by Chon, Seung-Hyun Jan 1, 2023 10:42pm
- This year, pharmaceutical bio companies' stock prices were sluggish. It has never recovered from its peak on the first day of the stock market opening. The market capitalization of major companies fell by more than 56 trillion won. More than two out of ten have seen their market capitalization shrink by less than half in a year. According to the
- Policy
- Australia, excluding drug price reference countries
- by Lee, Tak-Sun Jan 1, 2023 10:40pm
- Australia's addition to the drug price reference country, which faced opposition from the pharmaceutical industry, failed. The HIRA initially decided to take a step back from adding Australia and Canada to the drug price reference country and add only Canada to the reference country. The HIRA released the "Detailed Evaluation Standards for Dr
- Policy
- New formulations of narcotics will be as strictly reviewed
- by Lee, Jeong-Hwan Dec 30, 2022 06:33am
- Regulations on narcotics for medical use, such as narcotic appetite suppressants and propofol, which is used for general anesthesia, that the government is restricting new approvals for, are expected to become stricter than before. Until now, even narcotic medications for which new approvals are restricted by the government were allowed
- Policy
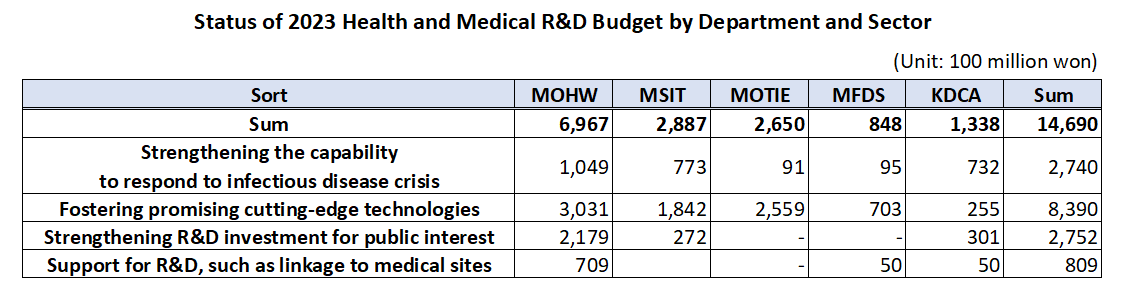
- Gov allocates KRW1.47 trillion budget for healthcare R&D
- by Kim, Jung-Ju Dec 30, 2022 06:33am
- The government’s budget for next year’s healthcare R&D including new drugs, medical devices, and digital transformation to AI-based biohealth is estimated to be around KRW 1.47 trillion. This is the total amount of budget that will be supported by the Ministry of Health and Welfare, Ministry of Science and ICT, Ministry of Trade, Industry, and
- Company
- Korea develops 2 new drugs and 2 biosimilars in 2022
- by Chon, Seung-Hyun Dec 29, 2022 06:04am
- In Korea, domestic pharmaceutical companies succeeded in developing 2 new drugs this year. The new drugs developed by SK Bioscience and Daewoong Pharmaceuticals reached the commercialization stage. This is the most amount of biosimilars developed in Korea in 7 years since 2015. ◆SK Bioscience receives approval for the first new homegrown
- Company
- EUA for Zochova was not requested
- by Kim, Jin-Gu Dec 29, 2022 06:04am
- The government also keeps the door open to "monitoring overseas situations"...U.S. and Europe are under review. Ildong Pharmaceutical is expected to shift its strategy of introducing oral COVID-19 treatment Zochova (s-217622) in Korea from the EUA to conditional permission. On the 28th, the Central Disease Control Headquarters announced th
- Policy
- A rapid change in the population
- by Kang, Shin-Kook Dec 29, 2022 06:03am
- Policy Tasks Determined at the Second Vice-Minister Meeting of the Ministry Related to Population Future Strategy. The government will come up with all-around measures due to population changes caused by the world's highest rate of low birth rate and aging society. This included institutionalization of non-face-to-face treatment and visiting med
- Policy
- Takeda CMV infection treatment Livtencity has been approved
- by Kim, Jung-Ju Dec 29, 2022 06:03am
- After transplantation of Takeda Pharmaceutical Korea, Cytomegalovirus infection treatment Livtencity obtained domestic item permission and passed the first gateway to supply. The Ministry of Food and Drug Safety (Director Oh Yoo-kyung) announced on the 27th that it has approved Livtencity of Takeda, a rare drug. Cytomegalovirus (CMV) is as
- Company
- Daewoong and Neurorive will codevelop new antidepressant
- by Lee, Seok-Jun Dec 29, 2022 06:03am
- On the 28th, Daewoong Pharmaceutical announced it had signed an agreement with Neurorive to conduct joint research and development for a new drug candidate for depression. Under the agreement, the two companies will be jointly developing ‘NR-0601,’ a multi-target, non-narcotic oral depression treatment. Neurorive is a bio venture com