- Company
- LegoChem Bio may receive milestone payments next yr
- by Dec 28, 2022 05:49am
- LegoChem Bioscience’s drug candidates that have been licensed out are making their way into clinical trials. The company succeeded in making licensing deals for 10 antibody-drug conjugate (ADC) pipelines and is expected to start receiving milestone payments in earnest in line with their development progress. ◆Company may earn up to
- Company
- More companies are overcoming patents for Otezla
- by Kim, Jin-Gu Dec 28, 2022 05:48am
- Pharmaceutical bio companies seeking to release generics for Otezla, a psoriasis treatment that has not been released in Korea, are expanding. In addition to the existing Daewoong Pharmaceutical and Dong-A ST, DongKoo Bio, Huons, and Chong Kun Dang succeeded in overcoming two out of three related patents. If they overcome the remaining usage
- Company
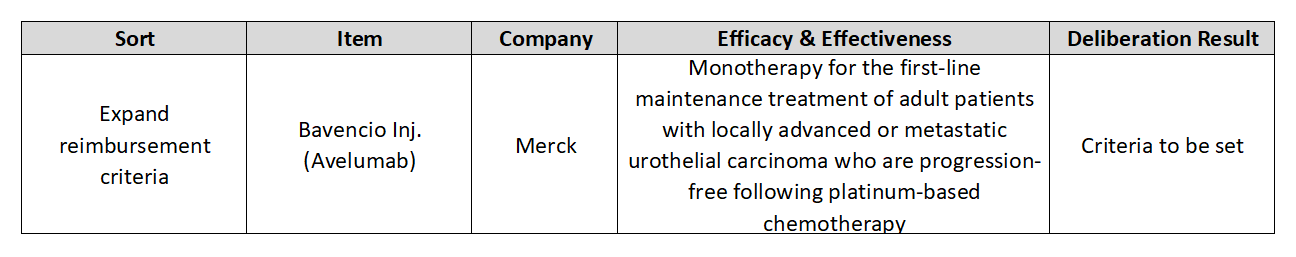
- Why no news is being heard on reimb extension for Bavencio
- by Dec 28, 2022 05:48am
- Merck and Pfizer’s cancer immunotherapy ‘Bavencio (avelumab)’ is having trouble expanding its reimbursement in Korea. Although the agenda has passed deliberations by the Cancer Disease Deliberation Committee (CDDC), the follow-up process was sluggish and no progress has been made for 8 months. According to the pharmaceutical industry on th
- Company
- Phase 1/2 of CJ Bio's Microbiome has been applied
- by Dec 28, 2022 05:48am
- CJ Bioscience announced on the 26th that it has submitted an IND of phase 1/2 of the Microbiome immuno-cancer drug "CJRB-101" to the U.S. Food and Drug Administration (FDA). CJRB-101 is a new immuno-cancer drug candidate secured by CJ Bioscience. It was developed through various immunological reviews based on the strain library that CJ has bu
- Company
- Kisqali Extends PFS by 1 yr in poor prognosis breast cancer
- by Dec 28, 2022 05:48am
- Novartis Korea announced on the 26th that the progressive and metastatic breast cancer treatment Kisqali has extended the progressive survival period by about one year compared to the first treatment of hormone receptor-positive (HR+)/human epithelial cell growth factor 2 negative (HER2-) breast cancer patients. The results of this study were
- InterView
- Avodart, Real World Data
- by Dec 28, 2022 05:48am
- GSK's large-scale real-world clinical results of Avodart, a treatment for prostatic hypertrophy, which marks the 13th anniversary of its launch in Korea, have been released for the first time. Based on actual field data, the company expressed its ambition to further strengthen Avodart's position in early hair loss treatment.LEAD clinical trials
- Policy
- Abbott’s Lipidil NT 145mg listed with reimbursement
- by Lee, Tak-Sun Dec 27, 2022 06:10am
- The original developer of the hyperlipidemia treatment fenofibrate will be releasing a 145mg product that can be taken on an empty stomach. This is the second drug to be released, following Yuhan Corp. As such, the two products are expected to compete fiercely in the market next year. According to industry sources on the 26th, Abbott
- Company
- Samsung BioLogics did not receive 45.5 billion won
- by Dec 27, 2022 06:10am
- Samsung BioLogics has been in conflict with U.S. bio company Cytodyn over the past year to pay for commissioned production (CMO). Conflicts have continued for a year as Cytodyn is overdue the promised cost of the biopharmaceutical CMO. Late payments more than doubled from $13.5 million (17.2 billion won) in January this year. According to the
- Product
- “Immediately stop enforcing the Healthcare Data Act”
- by Dec 27, 2022 06:10am
- Five major healthcare associations in Korea have called for the discontinuation of the enforcement of the Healthcare Data Act. The Korean Pharmaceutical Association (President: Kwang-Hoon Choi), Korean Medical Association (President: Pil-Soo Lee), Korean Hospital Association (President: Dong-Seop Yoon), Korean Dental Association (President: T
- Company
- LG Chem's Humira biosimilar applies for domestic permission
- by Dec 27, 2022 06:10am
- LG Chem announced on the 23rd that it has applied for permission for Humira biosimilar LBAL items to the Ministry of Food and Drug Safety. Indications include rheumatoid arthritis, psoriatic arthritis, axillary spinal arthritis, adult Crohn's disease, psoriasis, ulcerative colitis, Betchett enteritis, and meningitis in adults. In addition,