- Company
- Verzenio attempts reimb in early breast cancer in Korea
- by Eo, Yun-Ho Dec 20, 2022 06:06am
- The anticancer drug ‘Verzenio’ is attempting reimbursement listing for early breast cancer in Korea. According to industry sources, Lilly Korea has recently submitted an application for the reimbursement listing of its CDK4/6 inhibitor ‘Verzenio (abemaciclib)’ as a treatment for early-stage breast cancer with a high risk of recurrenc
- Policy
- Pfizer & Novartis generic drugs disappear from the market
- by Lee, Tak-Sun Dec 20, 2022 06:06am
- All generic drugs released by Novartis and Pfizer Korean branches in the domestic market have disappeared. Although it was successfully released, it is interpreted that it left the market after losing a lot of competition with domestic pharmaceutical companies. According to industries on the 19th, Novartis' Pneumast 10mg and Pneumast 3mg w
- Company
- MSD-Boryung Bio will copromote Prodiax 23 in Korea
- by Dec 20, 2022 06:05am
- On the 19th, MSD Korea announced it has selected Boryung Biopharma as its new distributor and supplier for its pneumococcal vaccine, ‘Prodiax 23.’ Under the agreement, Boryung Biopharma will be distributing and supplying both the private and contract Prodiax 23 products under the National Immunization Program starting January 1, 2023. M
- Policy
- President Yoon said, block medical shopping
- by Kang, Shin-Kook Dec 20, 2022 06:05am
- As concerns arose that it might lead to a reduction in health insurance coverage, President Yoon Suk Yeol mentioned a second plan to reform health insurance. Regarding the direction of health insurance reform at the first state affairs inspection meeting at the Blue House guesthouse on the 15th, President Yoon said, "It means that we will eli
- Company
- Shingrix presents a new paradigm for shingles prevention
- by Eo, Yun-Ho Dec 20, 2022 06:05am
- Korea GSK's herpes zoster vaccine "Shingrix" announced its official launch. GSK held a press conference at InterContinental Seoul COEX on the 15th to commemorate the launch of Shingrix, a shingles-prevention vaccine, in Korea. At the meeting, Yoon Kyung-young, an infectious medicine professor at Korea University Anam Hospital, introduced the
- Policy
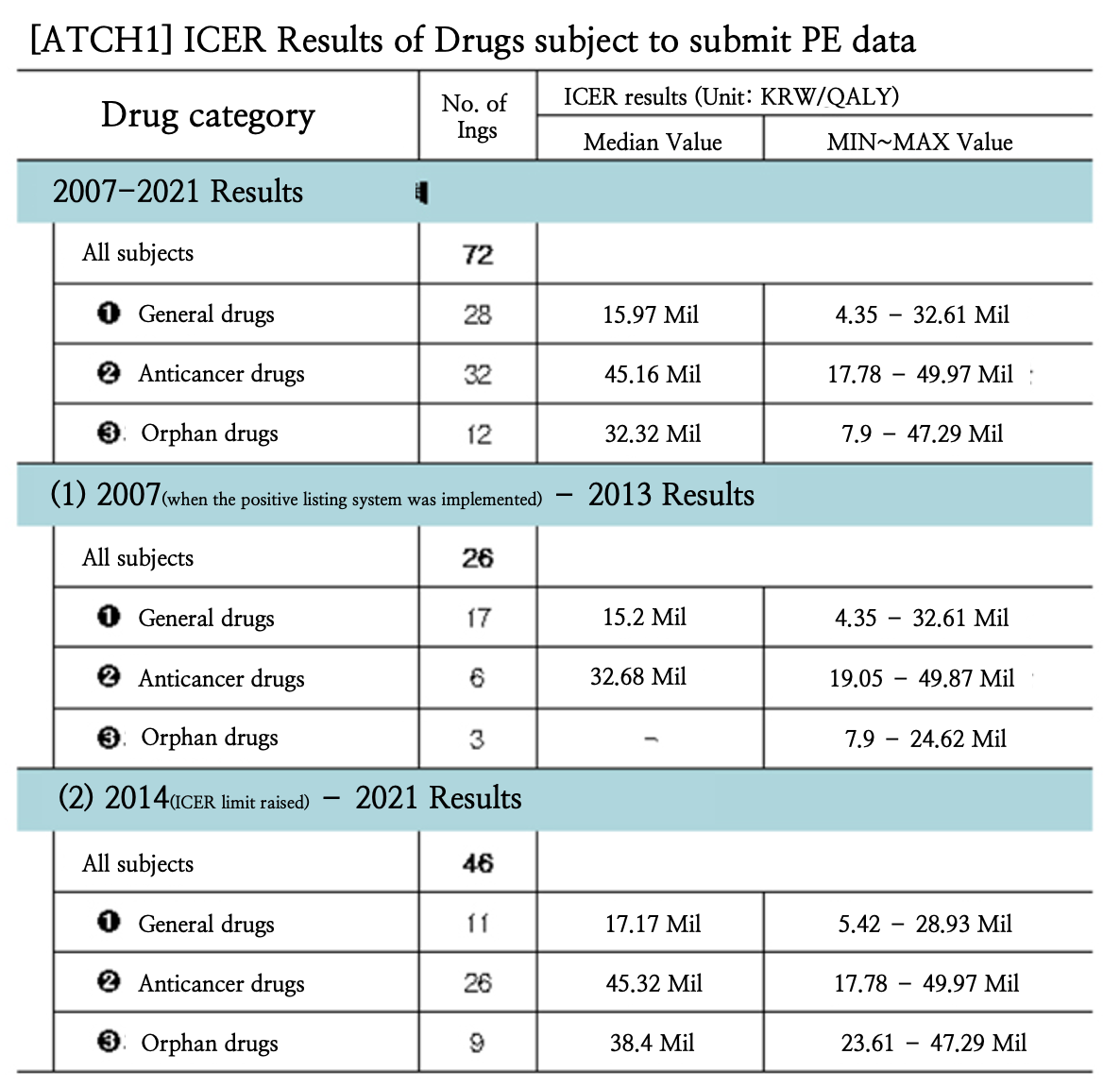
- ICER of general drugs KRW 15.97 mil for the past 15 yrs
- by Lee, Tak-Sun Dec 19, 2022 04:35am
- The median ICER (Incremental Cost-Effective Ratio) value of general drugs from 2007 to 2021 was KRW 15.97 million in Korea. The ICER value of anticancer drugs was KRW 45.16 million, and rare diseases KRW 15.97 million in the same period. This was the first time that the ICER results were disclosed, and the disclosed results are expected to be
- Policy
- Pfizer Cibinqo, re-applied to the HIRA
- by Lee, Tak-Sun Dec 19, 2022 04:35am
- Pfizer Cibinqo, which aims to pay for atopic dermatitis indications as a JAK inhibitor, is being paid later than expected. It was expected to be deliberated by the Drug Benefit Evaluation Committee within the year after passing the HIRA Drug Benefit Standards Subcommittee in August, but it is expected to take some time for the salary to be co
- Company
- Reimb for Revlimid as maintenance therapy near after 4 yrs
- by Eo, Yun-Ho Dec 19, 2022 04:35am
- After 4 long years of await, Revlimid as maintenance therapy is nearing reimbursement listing in Korea. According to industry sources, the agenda of reimbursing Revlimid as maintenance therapy that has passed deliberations by the Health Insurance Review and Assessment Service’s Cancer Disease Deliberation Committee in June will be delib
- Policy
- Green light for the reimb of Onureg, Rolontis, etc.
- by Lee, Tak-Sun Dec 19, 2022 04:35am
- A green light has been lit for the reimbursement of BMS Korea’s leukemia treatment ‘Onureg tab,’ with its agenda passing deliberations for setting reimbursement standards. Also, reimbursement standards have been prepared for 5 neutropenia treatment products including Hanmi Pharmaceutical’s Rolontis. The Health Insurance Review and Ass
- Policy
- HIRA, established a management system for high-priced drugs
- by Lee, Tak-Sun Dec 19, 2022 04:34am
- Medical institutions submit administrative information and response evaluation results to the Board of Audit and Inspection. The HIRA (Director Kim Sun-min) announced that it has been operating a "high-priced drug management system" since the 12th to increase the work efficiency of analyzing the results of response evaluations for patients r