- InterView
- “Will rise independently as Organon in the industry”
- by Eo, Yun-Ho Sep 8, 2022 05:59am
- Spin-offs, which are made for various reasons in various circumstances, bring out various positive and negative issues in the process. Organon’s course of the spin-off was also quite eventful. However, the company quickly straightened its affairs after completing the spin-off and being reborn as an independent organization in June last y
- Company
- Unilateral voluntary retirement
- by Sep 8, 2022 05:59am
- The GSK labor union of Korea has taken legal action, insisting on the management's unilateral voluntary retirement. The company is not taking any action, but the inside is "like the calm before the storm" as legal battles are predicted. Novartis Korea, where voluntary retirement is being carried out relatively quietly, is also nervous until t
- Policy
- Approval of Boryung’s new SCLC drug Zepzelca imminent
- by Lee, Hye-Kyung Sep 8, 2022 05:58am
- Boryung Pharmaceutical’s new drug for small-cell lung cancer (SCLC), 'Zepzelca inj, (lurbinectedin) is soon to be granted marketing approval in Korea. Currently, Hycamtin inj (topotecan)’ and ‘Camtobell Inj (belotecan)’ are approved for second-line use in Korea. According to industry sources on the 7th, the Ministry of Food and Dr
- Company
- Sales of immuno-cancer drugs exceeded 200 billion won
- by Sep 8, 2022 05:58am
- In the first half of this year, the domestic immune anticancer drug market surpassed 200 billion won. In particular, Opdivo's performance, which showed his endurance, was remarkable. Opdivo sales increased 41% from the previous year, surpassing 50 billion won in half-year sales. According to IQVIA, a pharmaceutical market research firm, th
- Company
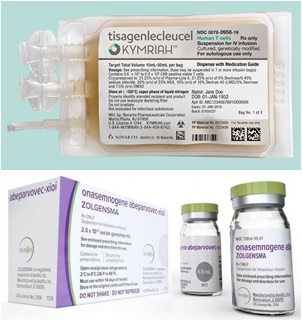
- Zolgensma, which is the same price as an apartment
- by Moon, sung-ho Sep 8, 2022 05:58am
- With ultra-high-priced treatments from global pharmaceutical companies newly entering the benefit range this year, human "risk management" is emerging as an issue at clinical sites. Since the treatment is so expensive, "risk" management issues such as damage and loss that may occur during administration are acting as a key issue. Kymriah,
- Policy
- MOHW to provide premium pricing support for innovative drugs
- by Lee, Jeong-Hwan Sep 7, 2022 05:52am
- The Korean government has emphasized the need to enact subordinate statutes to give preferential treatment to new drugs manufactured by Korea Innovative Pharmaceutical Companies and promises to prepare policy support. This announcement reflects the authorities’ determination to find a way to provide preferential treatment for drug prices
- Policy
- What's patient-centered safety in the biopharmaceutical era?
- by Lee, Hye-Kyung Sep 7, 2022 05:52am
- Along with the application of the world's first CAR-T cell therapy Kymriah and Zolgensma, an alternative treatment for spinal proximal phagocytosis (SMA), the biopharmaceutical era has opened in Korea. Controversy over safety and accessibility has not yet been resolved in the process of innovative biopharmaceuticals being developed and used to t
- Company
- Godex’s price to be cut further under PVA
- by Nho, Byung Chul Sep 7, 2022 05:52am
- Celltrion Pharm’s liver disease treatment Godex cap. is experiencing ‘double trouble,’ being subject to Price-Volume Agreement negotiations after reimbursement reevaluations. According to industry sources, Godex’s price will be cut by &8361;15 per capsule (4% reduction) from &8361;371 to &8361;356 as of the 1st of this month.
- Company
- Poziotinib responds 100% to pts with lung cancer mutations
- by Sep 7, 2022 05:52am
- Poziotinib, a new lung cancer drug exported by Hanmi Pharmaceutical, was found to have a significant effect on the G778 mutation, which is common in patients with HER2 Exxon20 insertion mutation. As a result of ZENITH20 clinical subanalysis, 12 patients showed 100% response, and PFS was also longer than other HER2 Exxon20 mutants. In particul
- Policy
- Antiemetic Emend's generic benefit after 15 yrs since origin
- by Lee, Tak-Sun Sep 7, 2022 05:51am
- As the generic of Emend will be applied starting this month, the burden of drug prices for patients is expected to decrease. It is the first time in 15 years that generic for Emend capsule has been reimbursed since the original was listed. According to industries on the 5th, Acepharma's Atant 80mg and Atant 125mg have been registered as be