- Company
- AstraZeneca Korea launches ‘Lung Health Checkbus’ campaign
- by Whang, byung-woo Jul 1, 2025 06:00am
- AstraZeneca Korea announced on the 30th that it held a ceremony for the launch of its ‘Lung Health Checkbus’ campaign at the COEX Square in Seoul on the 27th. The campaign aims to help people detect lung nodules that they are not aware of at an early stage by operating buses equipped with AI-based chest X-ray imaging nationwide. Ast
- Company
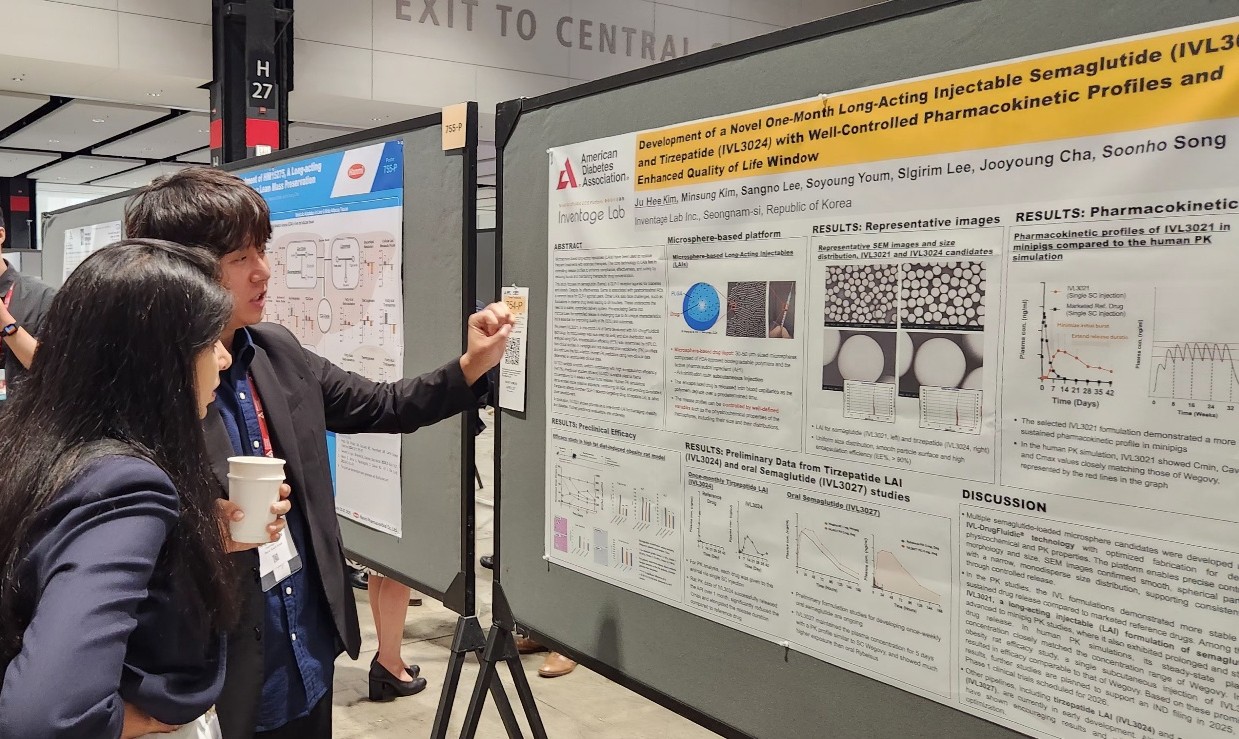
- New K-drugs for metabolic diseases make international debut
- by Son, Hyung Min Jun 30, 2025 06:07am
- Major Korean pharmaceutical and biotechnology companies have signaled their full-scale entry into global clinical trials, presenting new drug development results at overseas conferences. The companies presented their achievements in developing new drugs for various metabolic diseases, including obesity, type 2 diabetes, and metabolic dysfunction
- Company
- Adempas may be prescribed at general hospitals in KOR
- by Eo, Yun-Ho Jun 30, 2025 06:06am
- Adempas, a new treatment for pulmonary arterial hypertension that has emerged after a long wait, is now available for prescription at general hospitals in Korea. According to industry sources, Bayer Korea's Adempas (riociguat) has been approved by the Drug Committee (DC) of tertiary hospitals in Korea, including Samsung Medical Center and
- Opinion
- [Reporter's View] Divisional restructuring needed
- by Lee, Hye-Kyung Jun 30, 2025 06:06am
- During his candidacy, President Lee Jae-myung pledged to designate the biotech industry as a cutting-edge sector and build it as the future growth engine, aiming to position South Korea as one of the world's five strongest biotech countries. Based on an analysis that the previous government was short on investment in the pharmaceutical and bi
- Policy
- More generic 'Vimovo' drugs with naproxen+PPI enters the mkt
- by Lee, Tak-Sun Jun 30, 2025 06:05am
- Generic drugs containing the same active ingredients as the 'Vimovo' (naproxen+esomeprazole magnesium trihydrate), a combination of an anti-inflammatory drug and an anti-ulcer agent, are set to be released. A generic has not been approved since Chong Kun Dang's 'Naxen S Tab' was approved in 2024. Considering the characteristics of a generi
- Policy
- Polivy granted partial reimbursement after 5 years
- by Lee, Tak-Sun Jun 30, 2025 06:05am
- Roche’s Polivy (polatuzumab vedotin), a treatment for diffuse large B-cell lymphoma (DLBCL) that is currently non-reimbursed in Korea, has been added to the reimbursement list as a part of combination therapy. With the listing, the other drugs used in the combination, excluding Polivy, will be reimbursed. This measure is in accordance wi
- Opinion
- [Reporter's View] For local gov't seeking biotech diplomacy
- by Whang, byung-woo Jun 30, 2025 06:05am
- 'BIO USA,' held in Boston, U.S., is the world's largest biotech industry conference. Notably, this year's conference was attended by officials from Korea's local government. The attending officials aimed to promote local biotech clusters and seek potential investment partners. Through this event, they intended to establish a gamer-changer
- Company
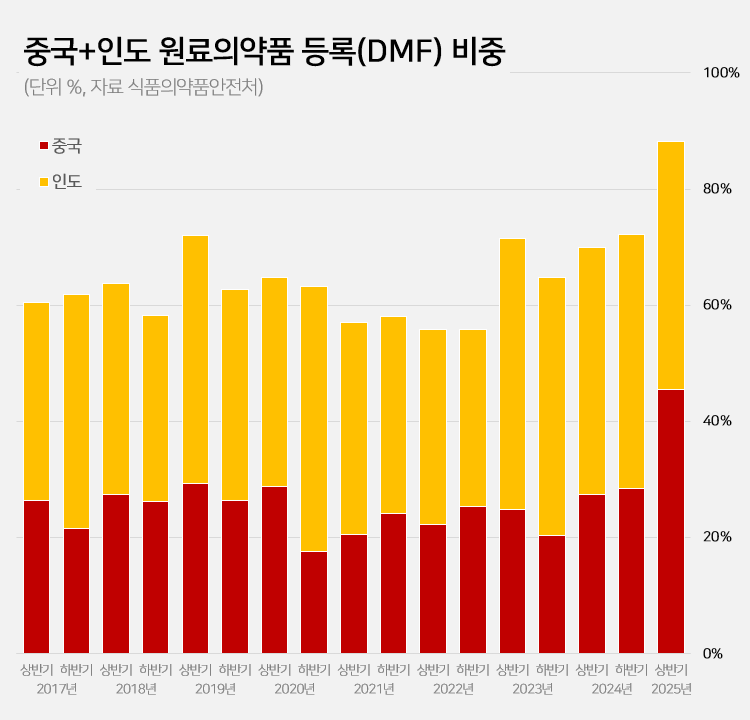
- 88% registered APIs imported from China or India
- by Kim, Jin-Gu Jun 27, 2025 06:04am
- Amid a surge in the number of drug master file registrations in the first half of this year, the share of raw materials from China and India rose to 88.2%. This is a sharp increase compared to the average of 62.1% share the two countries had during the past 5 years. This is attributed to the large number of previously delayed raw material dru
- Policy
- Ensuring stable supply of drugs in short supply
- by Lee, Jeong-Hwan Jun 27, 2025 06:03am
- Following President Lee Jae-myung's pledge to establish a stable supply system for drugs with supply shortages, attention is drawn to the Ministry of Health and Welfare's (MOHW) opinion that a social consensus on the criteria and scope of 'supply shortage' is first needed. It is anticipated that legislative review in the National Assembly
- Company
- Tevimbra adds esophageal, gastric, lung cancer indications
- by Whang, byung-woo Jun 27, 2025 06:02am
- BeiGene Korea (Name to be changed to BeOne Medicine Korea) announced that its immuno-oncology drug Tevimbra (tislelizumab) has been approved by the Ministry of Food and Drug Safety for additional indications for esophageal cancer, gastric cancer, and non-small cell lung cancer. With the additional approval, Tembriva can now be used as a fi