- Company
- KPTA ‘KOR-CHN-JPN supply cooperation to bring $12B effect'
- by Kim, Jin-Gu Jun 27, 2025 06:02am
- The Korea Pharmaceutical Traders Association (KPTA), China Chamber of Commerce for Import & Export of Medicines & Health Products (CCMPHIE), and Japan Pharmaceutical Traders Association (JPTA) announced on June 25 that they signed a memorandum of understanding (MOU) for the stabilization of the pharmaceutical supply chain at the Korea Pavi
- Company
- ‘Wegovy, a game-changer for high-risk obesity patients’
- by Whang, byung-woo Jun 26, 2025 06:08am
- Obesity is a cause of various metabolic syndromes and a major risk factor for cardiovascular disease. In fact, approximately 80% of patients hospitalized for cardiovascular disease are obese, and studies have shown that the risk of cardiovascular events in obese patients is up to twice as high as in those of normal weight. Over the past 20 ye
- Company
- The 2nd KRAS-targeted cancer drug 'Krazati' expected
- by Eo, Yun-Ho Jun 26, 2025 06:08am
- The second KRAS inhibitor is expected to be commercialized in South Korea. Bristol Myers Squibb (BMS) Korea recently submitted a marketing authorization application to the Ministry of Food and Drug Safety (MFDS) for its anti-cancer drug, Krazati (adagrasib). Krazati was also designated as an orphan drug in January. It is indicated for
- Product
- 'Govn’t must take strong action against pharma rebates'
- by Kang, Hye-Kyung Jun 26, 2025 06:08am
- The Korean Pharmacists for Democratic Society (President Gyeong-rim Jeon, KPDS) has urged the government to take strong action against pharmaceutical company rebates. KPDS issued a statement regarding rebates made by a major domestic pharmaceutical company that JTBC reported. In the statement, KPDS said, “This is not an individual incide
- Policy
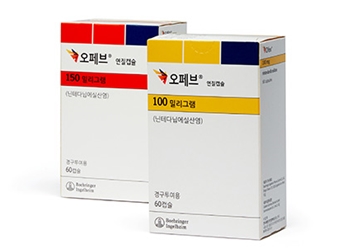
- Yungjin, Ildong’s Ofev generics enter market at half price
- by Lee, Tak-Sun Jun 26, 2025 06:07am
- A generic version of Ofev (nintedanib), a treatment for chronic fibrotic interstitial lung disease), will enter the market at half the price of the original drug. With the entry of generic drugs, the Ofev market now faces competition, just two months after the original drug was listed for reimbursement. According to industry sources o
- Company
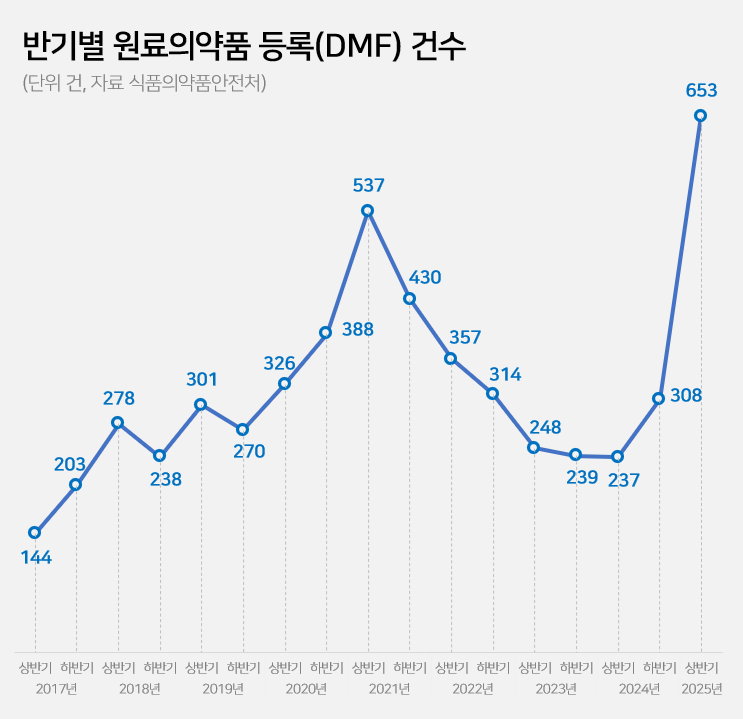
- Drug Master File for API 237→653…'easing of regulation'
- by Kim, Jin-Gu Jun 26, 2025 06:07am
- The number of Drug Master File, DMF, cases in the first half of this year surged by 2.8 times compared to the same period last year. This is the highest for a half-year period. Analysis suggests that this is due to the easing of Active Pharmaceutical Ingredient (API) registration requirements at the beginning of the year. The government had
- Policy
- Daewoong's high-dose generic 'Xeljanz' wins nod
- by Lee, Hye-Kyung Jun 26, 2025 06:06am
- Daewoong Pharmaceutical's oral generic version of 'Xeljanz (tofacitinib),' which is used to treat rheumatoid arthritis, received approval. On June 23, the Ministry of Food and Drug Safety (MFDS) approved Daewoong Pharmaceutical's two dosages of 'Xeltofa Tab': 5 mg and 10 mg. The latest approval grabs attention, particularly because Dae
- Opinion
- [Reporter's View] Silent efforts pay off in the market
- by Son, Hyung Min Jun 25, 2025 06:02am
- In the first half of this year, South Korean pharmaceutical and biotech companies achieved notable success through technology exports. A series of major licensing deals were signed based on the companies’ innovative drug development platforms and clinical trial results the companies steadily developed and accumulated over the years. In A
- Policy
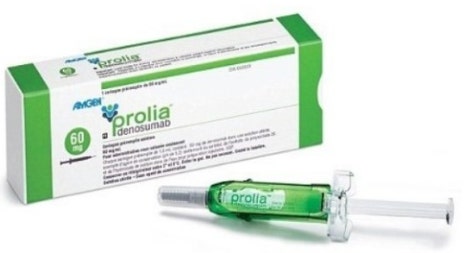
- Samsung Bioepis joins Prolia market with Obodence
- by Lee, Tak-Sun Jun 25, 2025 06:02am
- With Samsung Bioepis entering the biosimilar market for Prolia (denosumab), competition is expected to intensify among co-promoter companies Daewoong Pharmaceutical (Stoboclo, Celltrion), Hanmi Pharmaceutical (Obodence, Samsung Bioepis), and Chong Kun Dang (original Prolia, Amgen). Chong Kun Dang signed a co-promotion agreement with the origi
- Company
- Vemlidy indication extended to children aged 6 and older
- by Whang, byung-woo Jun 25, 2025 06:01am
- Gilead Sciences Korea announced on the 24th that its hepatitis B treatment Vemlidy (tenofovir alafenamide, TAF) has been approved by the Ministry of Food and Drug Safety for the treatment of chronic hepatitis B in children aged 6 years and older. Vemlidy offers improved renal and bone safety compared to existing chronic hepatitis B treatm