- Company
- Sanofi’s Allegra dominates OTC·ETC drug market
- by Nho, Byung Chul Jun 24, 2022 05:46am
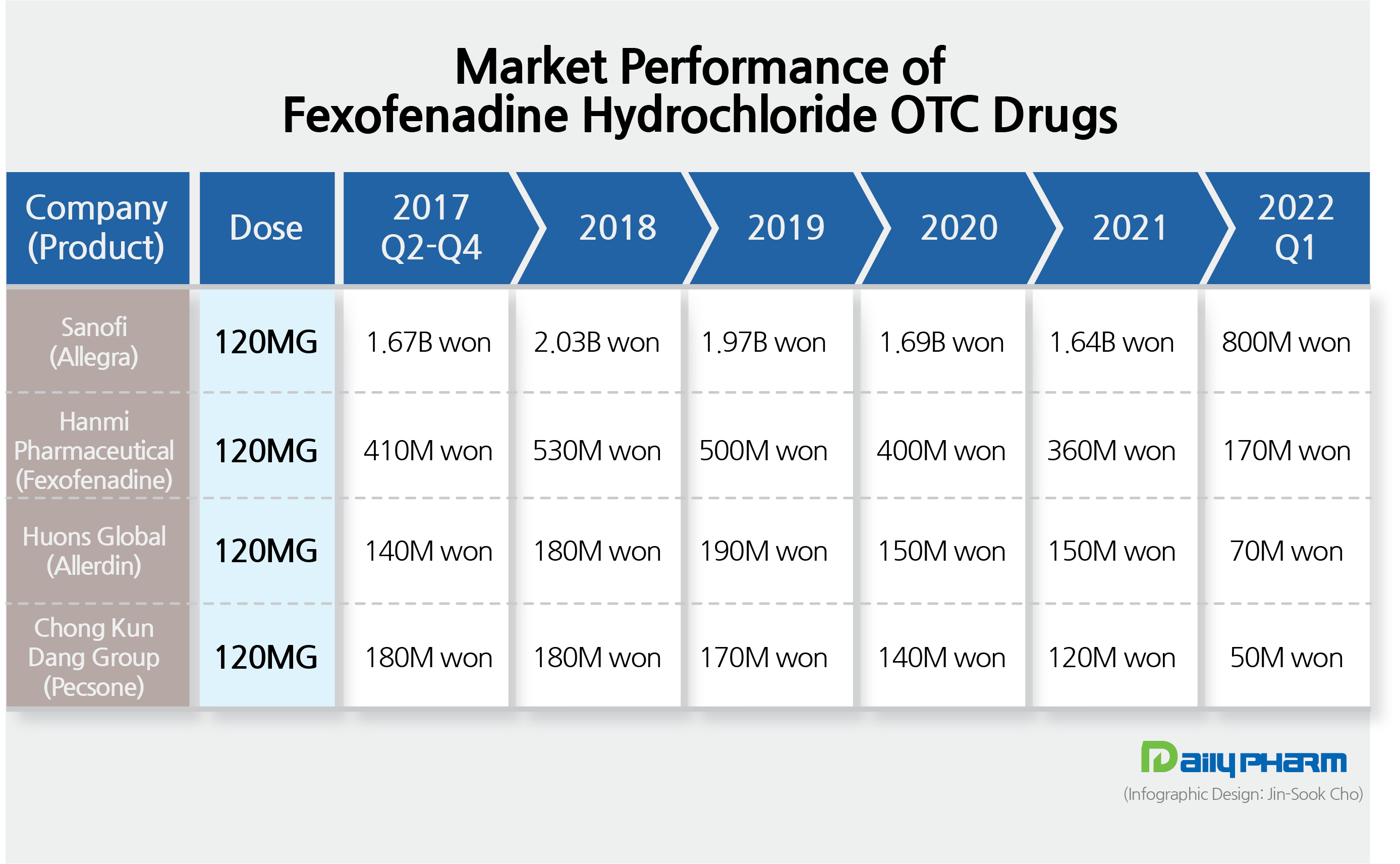
- The market for OTCs and ETCs of the antihistamine ingredient fexofenadine hydrochloride has been showing stagnant performance with its sales making a rectangle pattern for several years now. The market is virtually monopolized by the original drug, Sanofi’s Allegra Tab., with the total market estimated to be in the &8361;8 billion range
- Company
- Yuhan’s new lung cancer drug Leclaza shows OS benefit
- by Nho, Byung Chul Jun 24, 2022 05:46am
- Yuhan Corp (CEO and President Wook Je Cho) announced on the 23rd that it had confirmed the overall survival (OS) benefit of Leclaza (lasertinib) in the Phase I/II LASER201 trial (NCT03046992). Leclaza is a treatment for epidermal growth factor receptor (EGFR) T790M mutation-positive non-small cell lung cancer (NSCLC). Leclaza is a third-g
- Policy
- A suspected Monkeypox patient entered Korea
- by Lee, Jeong-Hwan Jun 24, 2022 05:46am
- With two suspected Monkeypox infections confirmed to have entered Korea, President Yoon ordered accelerated approval of vaccines and antiviral drugs from quarantine authorities. On the 22nd, President Yoon ordered, "Strengthen the management of entry and quarantine through airports and closely monitor the additional occurrence in Korea." C
- Policy
- Abnormal cases of Inlyta in PMS for 9 yrs is 82.8%
- by Lee, Hye-Kyung Jun 24, 2022 05:45am
- As a result of a post-marketing survey of Korea Pfizer Pharmaceutical's kidney cancer treatment Inlyta, the incidence of abnormal cases was 82.88% regardless of the causal relationship. As a result of conducting PMS on 111 people over 9 years for re-examination, 92 people (338 cases in total) showed abnormal cases regardless of causality.
- InterView
- Tremfya to bring generation shift in the IL inhibitor market
- by Jun 23, 2022 05:50am
- The competition among latecomers is intensifying in the interleukin inhibitor market with the scope of their indications expanding to psoriatic arthritis. The leader in this market is Janssen’s IL-12/23 inhibitor, ‘Stelara (Ustekinumab).’ Although 10 years have passed since its approval, the drug still boasts a growth rate in the 30% range
- Opinion
- [Reporter's view] Only 2% of patients
- by Eo, Yun-Ho Jun 23, 2022 05:50am
- Will the new EGFR Exon 20 Insertion-Mutated Non-Small-Cell Lung Cancer treatment, which was useless for existing TKI, be able to receive insurance benefits? It's all the same cancer, but it's different. Cancer species such as liver cancer, stomach cancer, and lung cancer, which we call, are only a simple category, and in fact, they are class
- Company
- LG Chem’s Zemiglo+Forxiga combo Zemidapa is approved
- by Chon, Seung-Hyun Jun 23, 2022 05:50am
- LG Chem made a public announcement on the 22nd that the company had received marketing authorization for its type 2 diabetes treatment ‘Zemidapa Tab’ from the Ministry of Food and Drug Safety. The drug is a fixed-dose combination of the antidiabetic drug gemigliptin and dapagliflozin. Gemigliptin is an active ingredient of "Zemiglo," a
- Company
- Hyundai Introduces Estrogen-Free Contraceptives
- by Jun 23, 2022 05:50am
- Hyundai Pharmaceutical (CEO Lee Sang-joon) announced on the 21st that it has signed an exclusive license agreement with Asuka Pharmaceutical in Korea for the oral contraceptive Slinda"with Drospirenone only. Slinda is a product developed by Exeltis, a women's medical brand division in Insud Pharma, Spain. Unlike the existing fourth-genera
- Product
- Approval of OTC teleconference bending machine project
- by Kang, Shin-Kook Jun 23, 2022 05:49am
- OTC teleconference bending machine issue, which has been on hold for 10 years, has secured a bridgehead for entering the market under the new government deregulation stance. The Ministry of Science and ICT held an ICT regulatory sandbox review committee on the 20th and approved the OTC teleconference bending machine based on the MOHW's co
- Policy
- Kadcyla to recieve reimb in early breast cancer from July
- by Lee, Tak-Sun Jun 22, 2022 05:59am
- Roche Korea’s Kadcyla inj. (ado-trastuzumab emtansine) is expected to additionally receive insurance benefits for early breast cancer in Korea. Kadcyla, a breast cancer treatment that Roche released to succeed Herceptin, has been limitedly used in patients with locally advanced or metastatic breast cancer in Korea until now. The Health