- Policy
- Handok and JW’s new imported drugs reimbursed from July
- by Lee, Tak-Sun Jun 23, 2025 06:00am
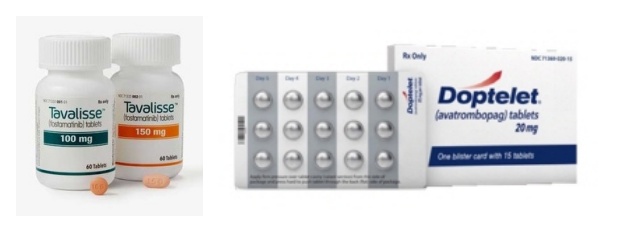
- In July, four new drugs, including an oral drug for paroxysmal nocturnal hemoglobinuria (PNH), a new biological agent for severe atopic dermatitis, and a new drug for immune thrombocytopenia (ITP), will be reimbursed by health insurance and be available with coverage for patients. On the 19th, the MOHW announced the establishment of reim
- Company
- "6-in-1 vaccine Hexaxim may enhance preventive effect of NIP
- by Whang, byung-woo Jun 23, 2025 06:00am
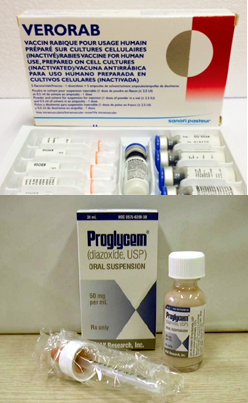
- "Hexaxim is Korea's first hexavalent vaccine. It is expected to play a crucial role in helping protect children's health." Following the inclusion of Hexaxim, a hexavalent (6-in-1) DTaP vaccine, in the National Immunization Program (NIP) this year, the medical field is holding high hopes for increased convenience of immunization and improveme
- Policy
- Rapid adjustment of insurance prices for rare drugs granted
- by Lee, Hye-Kyung Jun 20, 2025 06:08am
- The insurance price ceiling adjustment term for insured drugs supplied by the Korea Orphan Drug Center (KOEDC) is expected to be significantly reduced from the initial 8-9 months to around 4 months. Previously, it took at least 8-9 months to adjust the price cap, raising concerns about supply disruptions and financial losses for the cente
- Company
- Cream JAKi Delgocitinib to be commercialized in Korea
- by Eo, Yun-Ho Jun 20, 2025 06:06am
- JAK inhibitors will soon be available for hand eczema in Korea as well. The Ministry of Food and Drug Safety is currently conducting a final review of LEO Pharma Korea's treatment for moderate-to-severe chronic hand eczema (CHE), Anzupgo (delgocitinib). When approved, the drug is expected to be officially commercialized in the second half
- Policy
- SGLT2 Jardiance likely to receive expanded reimb for CKD
- by Lee, Tak-Sun Jun 20, 2025 06:06am
- The SGLT-2 inhibitor Jardiance (empagliflozin, Boehringer Ingelheim) is expected to be reimbursed for the treatment of chronic kidney disease (CKD). This drug is currently reimbursed for the treatment of diabetes and chronic heart failure. If expanded reimbursement is approved for CKD, the volume of usage is likely to be increased. Further
- Company
- Interest in Lotte Biologcs’ partner Ottimo Pharma rises
- by Kim, Jin-Gu Jun 20, 2025 06:05am
- Interest is growing in Ottimo Pharma, a biotech company that has signed a contract manufacturing agreement for its antibody-drug with Lotte Biologics. Founded in 2017, this UK-based biotech venture owns a new drug candidate called ‘Jankistomig’. The drug candidate bifunctional antibody targeting solid tumors, and the company plans to s
- Company
- New pneumococcal vaccine expected to be launched
- by Whang, byung-woo Jun 19, 2025 06:04am
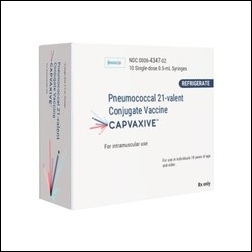
- As 'Capvaxive,' a 21-valent pneumococcal conjugate vaccine (PCV21) developed by MSD, is anticipated to receive marketing authorization in South Korea, competition in the market is likely to heat up. According to pharmaceutical industry sources, MSD has filed with the Ministry of Food and Drug Safety (MFDS) for marketing authorization of Ca
- Company
- K-Bios face string of clinical failures in H1
- by Son, Hyung Min Jun 19, 2025 06:04am
- A series of clinical trial failures for new drug candidates under development by Korean biotech companies in the first half of the year have raised concerns about the feasibility of future technology exports. Orum Therapeutics halted a clinical trial due to safety concerns, while Genexine and Bridge Biotherapeutics both failed to demonstrate sta
- Company
- KOR-JPN jointly launches Healthcare Distribution Alliance
- by Son, Hyung Min Jun 19, 2025 06:03am
- Three pharmaceutical distribution companies in Korea and Japan have joined forces to launch the Healthcare Distribution Alliance to lead the domestic market by introducing advanced overseas models. The alliance aims to go beyond simple logistics agreements &8211; it seeks to build an innovative cooperation structure where companies can shar
- Policy
- Growing role of the Korea Orphan & Essential Drug Center
- by Lee, Hye-Kyung Jun 19, 2025 06:03am
- "The emergency import of essential medicines through the Korea Orphan & Essential Drug Center (KOEDC) will be expanded, and support for pharmaceutical companies producing domestic products will be planned." President Lee Jae-myung made this pledge on his Facebook page on May 28, during his campaign for the presidency. President Lee emphasized