- Policy
- Phase III for development of Alecensa & Keytruda
- by Lee, Hye-Kyung Apr 26, 2022 06:11am
- Roche Korea and MSD Korea are accelerating clinical trials to develop combination therapy for non-small cell lung cancer treatments. On the 22nd, the MFDS approved phase 3 clinical trials of MSD's Keytruda and Keytruda SC and Roche's Alecensa. All of these pharmaceutical companies begin clinical trials to confirm the safety and effectiv
- Policy
- Exclude drugs that have increased due to COVID-19 from PVA
- by Lee, Tak-Sun Apr 25, 2022 06:08am
- It is reported that the pharmaceutical industry plans to officially propose to the government to exclude respiratory treatments that have increased their use due to the increase in COVID-19 patients from PVA. It is asked to reflect that the explosive increase in the use of the drug is inevitable due to the increase in the number of patients w
- Company
- JAKi options added to Dupixent for atopic dermatitis
- by Eo, Yun-Ho Apr 25, 2022 06:07am
- Two new treatment options were added to the field of atopic dermatitis that had only Dupixent as an option. The new options are both JAK inhibitors. The Ministry of Health and Welfare had issued a pre-administrative notice on the ‘Details on the standard and methods for applying long-term care benefits (pharmaceuticals)’ and announced i
- Policy
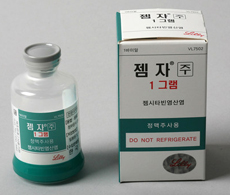
- Boryung’s Gemzar switches to domestic production
- by Lee, Hye-Kyung Apr 25, 2022 06:07am
- In 2 years since Boryung Pharmaceutical acquired the domestic rights for Lilly’s anticancer treatment ‘Gemzar (gemcitabine HCl)’ in Korea, the company switched all its items to domestic productions. According to the Ministry of Food and Drug Safety, Boryung Pharmaceutical withdrew its import license for ‘Gemzar’ on the 21st, and swit
- Policy
- Chong Kun Dang salt modified Entresto will be released soon
- by Lee, Hye-Kyung Apr 25, 2022 06:07am
- The market launch of Sacubitril/Valsartan Calcium, developed by Chong Kun Dang is imminent. According to the pharmaceutical industry on the 21st, Chong Kun Dang filed an application with the MFDS for permission for the drug developed by changing Valsartan Sodium, the main ingredient of Novartis' chronic heart failure treatment Entresto.
- Company
- COVID-19 Tx Lagevrio lands in tertiary hospitals in Korea
- by Eo, Yun-Ho Apr 25, 2022 06:07am
- The oral COVID-19 treatment ‘Lagevrio’ has officially landed for prescriptions at general hospitals in Korea. According to industry sources, MSD Korea’s Lagevrio (molnupiravir) has passed the drug committees of tertiary hospitals including the Seoul National University Hospital in Korea. With the approval, the drug may be prescribed fo
- Company
- Sales of Expectants & antibiotic have increased
- by Chon, Seung-Hyun Apr 25, 2022 06:07am
- Sales in the outpatient prescription market have been rising sharply this year. Sales in the prescription market have been rising as the number of COVID-19 confirmed cases has soared since late last year. The Expectant and antibiotic prescription market, which is used for colds and infectious disease diseases, recovered to the level before the o
- Company
- LDL-C target for cardiovascular dz will be lowered in Korea
- by Apr 25, 2022 06:07am
- The LDL-C target figure for South Korea's ultra-high-risk group for cardiovascular diseases is expected to be lowered to a similar level to global guidelines. The Korean Society of Lipid and Atherosclerosis held the Spring Cardiovascular Integration Academic Conference online and offline at the HICO on the 15th and 16th and unveiled some of t
- Policy
- Expansion of benefit standards such as Tenofovir/Baricitinib
- by Kim, Jung-Ju Apr 24, 2022 06:33pm
- As the scope of benefit of registered drugs such as chronic hepatitis B oral drug Vemlidy expands, the benefit standards for these drugs will also be expanded and changed. In addition, the standards for Baricitinib PO such as oluminant 2mg will be expanded to patients with chronic severe atopic dermatitis. The MOHW unveiled the "revise
- Company
- Samsung BioLogics acquired all of its shares in Epis
- by Chon, Seung-Hyun Apr 24, 2022 06:33pm
- Samsung BioLogics will incorporate Samsung Bioepis as a 100% subsidiary and operate a separate management system. Samsung BioLogics announced on the 20th that it has completed the first payment of $1 billion in the acquisition of Epis shares to Biogen. From the day the first payment was completed under the contract between the two companies,