- Company
- Imbruvica fails CDDC twice and reattempts 1st-line reimb.
- by Eo, Yun-Ho Apr 5, 2022 05:59am
- Once again, the blood cancer drug ‘Imbruvica’ is attempting to expand reimbursement to first-line treatment. According to industry sources, Janssen Korea has applied for the reimbursement extension of its Imbruvica (ibrutinib) as a first-line treatment for Chronic Lymphocytic Leukemia (CLL) and Small Lymphocytic Leukemia (SLL) to once
- Opinion
- [Reporter's view] Commercialization of innovative new drugs
- by Lee, Tak-Sun Apr 5, 2022 05:58am
- iLast month, immuno-cancer drug Keytruda was listed as the primary treatment for non-small cell lung cancer patients, and this month, the first chemical antigen receptor-T cell therapy (CAR-T) Kymirah received insurance benefits. Innovative treatments using patient immunity have succeeded in commercializing them in Korea one after another. The
- Product
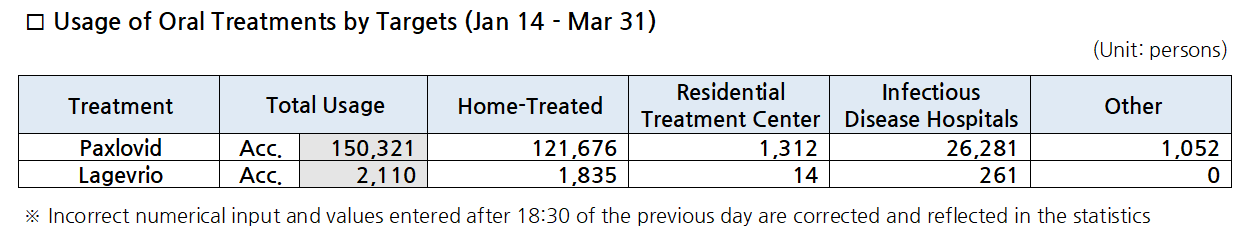
- Lagevrio prescriptions exceed 2,000… Paxlovid's up 25.6%
- by Kang, Shin-Kook Apr 5, 2022 05:58am
- The number of oral COVID-19 treatment Largevrio’s prescriptions has exceeded 2,000 in only 5 days since it was supplied to pharmacies in Korea. Also, the number of total Paxlovid prescriptions has exceeded 150,000 courses. According to the Korea Disease Control and Prevention Agency on the 1st, a total of 150,321 prescriptions were made fro
- Policy
- Kymriah has been listed, hope of cure
- by Lee, Jeong-Hwan Apr 5, 2022 05:57am
- Patients immediately welcomed the confirmation of health insurance benefits of the acute lymphocytic leukemia and lymphoma CAR-T treatment Kymriah (Tisagencleucel). The patients urged the transition committee of the next presidential post and Yoon Seok-yeol to quickly register new drugs directly related to life as health insurance benefits
- Company
- AZ starts next-gen ADC dev with confidence from Enhertu
- by Apr 4, 2022 06:07am
- AstraZeneca is leading the next-generation ADC development environment. The company, which has successfully commercialized ‘Enhertu’ in partnership with Daiichi Sankyo, is targeting areas slow in development such as triple-negative breast cancer. On the 31st, AstraZeneca received approval for its Phase III trial of a new ADC drug last
- Policy
- Phase 3 of Jetema the toxin will be conducted in Korea
- by Lee, Hye-Kyung Apr 4, 2022 06:07am
- Jetema the toxin 100U, which has been approved for export botulinum toxin, will conduct phase 3 clinical trials in Korea. The MFDS recently approved Jetema the toxin for a "multi-organization, double-blind, random assignment, parallel design, noninferiority trials, active control groups, and phase 3 clinical trials" for JTM201. The clinica
- Policy
- 20 ultra-high-priced drugs over ₩5 mil sold in Korea
- by Lee, Tak-Sun Apr 4, 2022 06:07am
- Survey results have shown that 20 high-priced drugs over &8361;5 million are approved for reimbursement 10 years after, Soliris, which had been then the most expensive drug in the world, was listed for reimbursement in Korea With ‘Kymriah,’ the one-shot treatment that was listed for reimbursement on April 1st, recording the highest pri
- Company
- Keytruda, the primary treatment for esophageal cancer
- by Apr 4, 2022 06:07am
- Keytruda, an immuno-cancer drug of MSD, has become the primary option in esophageal cancer, where treatments have been limited. The medical team predicted that an immuno-cancer drug-oriented treatment strategy using Keytruda or Opdivo will be established depending on the PD-L1 expression rate. At a seminar to commemorate the expansion of Keytrud
- Policy
- Based on PVA exclusion, the arithmetic average is 90%
- by Lee, Tak-Sun Apr 4, 2022 06:07am
- Drugs subject to PVA with an arithmetic average of less than 90% of the same product group are excluded. Previously, only drugs below the arithmetic average were excluded, but the target was further narrowed to less than 90%. Products with annual claims of less than 2 billion won are also excluded from PVA drugs. Previously, products worth
- Policy
- To commercialize innovative new drugs/supply essential drugs
- by Lee, Jeong-Hwan Apr 3, 2022 04:25pm
- The MFDS reports discuss ways to become a bio-health powerhouse The Presidential Acquisition Committee Yoon Seok-yeol and the MFDS agreed to systematically support the commercialization of high-tech and innovative medical products and establish a stable supply environment for rare essential drugs with low profitability. The transition com