- Policy
- To establish a pharmaceutical bio-innovation committee
- by Lee, Jeong-Hwan Mar 3, 2022 06:00am
- Yoon Suk-youl, presidential candidate for the presidential election, pledged to establish a "pharmaceutical bio-innovation committee" directly under the Prime Minister, while creating an ecosystem that can establish sovereignty in pharmaceutical bio and foster key talents and jobs in the pharmaceutical bio industry. It is said that it will ov
- Company
- Omicron confirmed cases continue to occur in companies
- by Ji Yong Jun Mar 2, 2022 05:55am
- Due to the spread of COVID-19 Omicron, there are a series of in-house confirmed cases within pharmaceutical bio companies. Pharmaceutical bio companies are responding by operating the Omicron emergency system and granting additional telecommuting to close contacts. According to the Central Disease Control Headquarters on the 2nd, the numbe
- Company
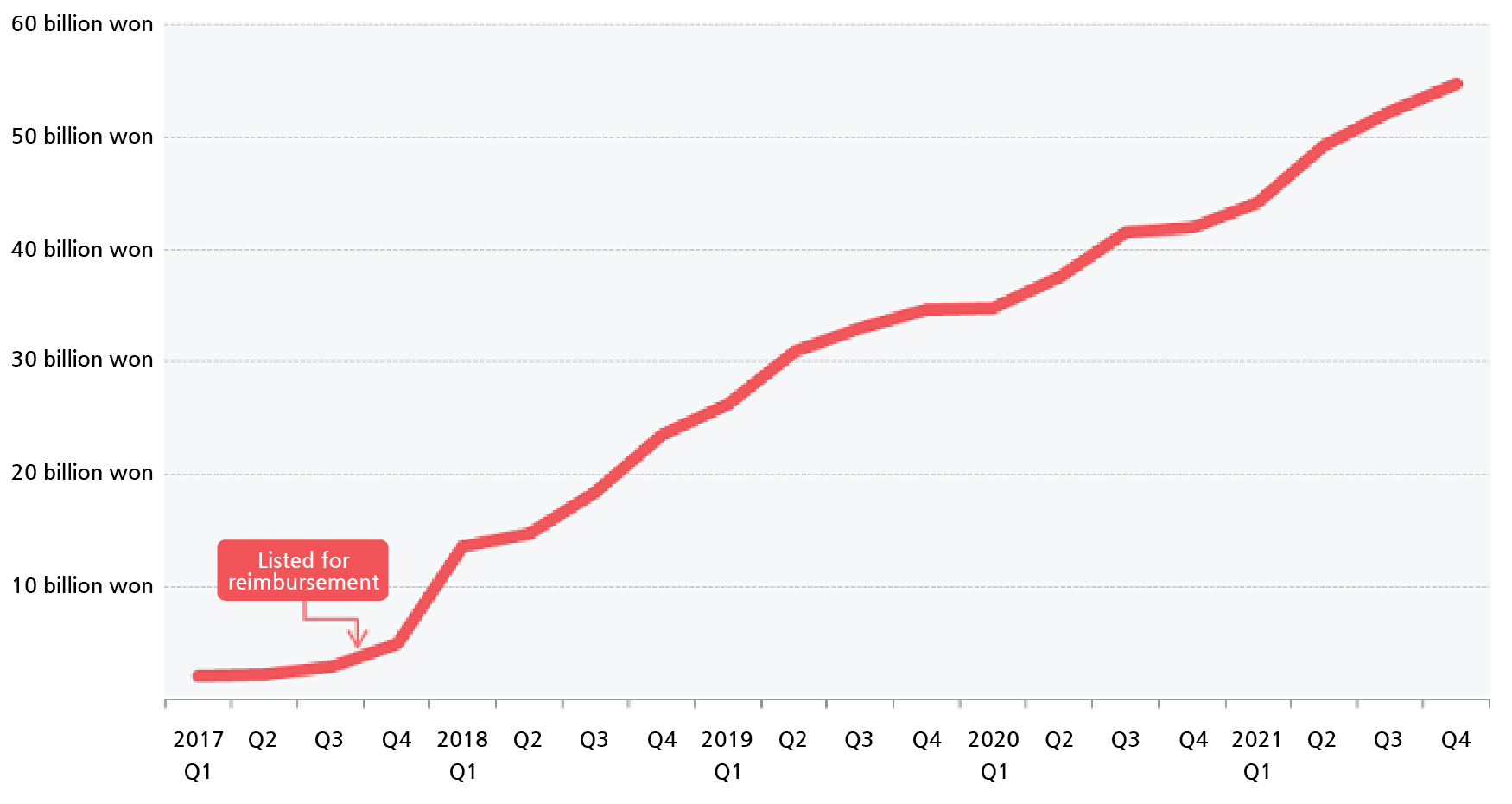
- Keytruda may make ₩300 billion with 1st-line reimb
- by Mar 2, 2022 05:55am
- The reimbursement expansion approval of Keytruda is expected to add wings to the sales growth of an already leading product in the domestic pharmaceutical market. It is expected that the company may achieve &8361;300 billion in sales with its reimbursement expansion to the first-line, which has more patients, from the &8361;200 billion sol
- Company
- Tylenol's sales amounted to ₩83.1 billion last year
- by Chon, Seung-Hyun Mar 2, 2022 05:54am
- OTC Tylenol recorded the highest sales ever. Sales more than doubled last year from the previous year due to a surge in demand for COVID-19 vaccinations. However, growth slowed in the second half of last year as demand for Tylenol was resolved. According to IQVIA, a pharmaceutical research institute, sales of the Tylenol series last year amou
- Policy
- Negotiations with NHIS on Actemra have been completed
- by Lee, Tak-Sun Mar 2, 2022 05:54am
- Actemra (Tocilizumab), a treatment for rheumatoid arthritis by JW Pharma, has been officially recognized as a treatment for COVID-19 in Korea. The drug, which has been used in patients with severe COVID-19 for purposes other than permission, will also be covered by health insurance benefits from March. On the 24th, the MOHW announced that
- Opinion
- [Reporter’s View] Why Big Pharmas 'select and focus'
- by Mar 2, 2022 05:54am
- In the famous TV show ‘Backstreet,’ CEO Baek Jong-won’s main advice and solution for small eateries suffering from slow business was to ‘reduce menus.’ Paik tells the restaurant owners to concentrate on their main menus instead of trying everything. Due to his consistent act of reducing menus in every restaurant he consults, he was fond
- Company
- Pfizer's 3rd attempt to reimburse ATTR-CM drug ‘Vyndamax'
- by Eo, Yun-Ho Feb 28, 2022 05:55am
- The third attempt at reimbursement for Vyndamax, a new drug for transthyretin amyloid cardiomyopathy, has been made by its company. According to industry sources, Pfizer Korea had again applied for the insurance reimbursement of its new drug that is indicated for the treatment of transthyretin amyloid cardiomyopathy (ATTR-CM). This is t
- Company
- Osteoporosis should be taken care of for the rest of life
- by Feb 28, 2022 05:55am
- The Korean Society for Bone and Mineral Research has begun to improve awareness of osteoporosis treatment. This is to enable continuous treatment by recognizing the seriousness of diseases that can lead to death from fractures and improving standards. Osteoporosis is a disease in which holes are formed in bones, and when bone strength weak
- Policy
- Keytruda is reimbursed as first-line at ₩2,107,642
- by Kim, Jung-Ju Feb 28, 2022 05:54am
- The insurance price of MDS Korea’s immuno-oncology drug Keytruda(pembrolizumab) inj. that is used for non-small-cell lung cancer and Hodgkin lymphoma will drop 25.6% with its reimbursement extended from the second-line to the first-line starting next month, Astellas Korea’s Xospata 40mg (gilteritinib), as well as Novartis Korea’s Lutather
- Policy
- BeiGene's Brukinsa has been approved in Korea
- by Lee, Hye-Kyung Feb 28, 2022 05:54am
- Brukininsa 80mg (Zanubrutinib), a BTK inhibitor from Chinese pharmaceutical company BeiGene, has obtained an item license in Korea. It is the second new drug approved by a Chinese pharmaceutical company after Antengene's Xpovio 20mg (Selinexor) in July last year. On the 24th, the MFDS approved Brukinsa, a new drug for treating blood cancer