- Policy
- The benefit standard for Braftovi & Lorviqua was set
- by Lee, Hye-Kyung Jan 19, 2022 06:06am
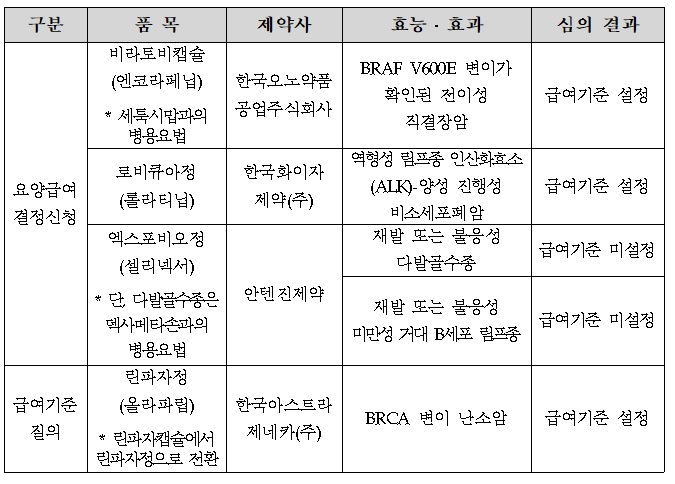
- At the first Severe (Cancer) Disease Review Committee held this year, the benefit standards for Braftovi, a direct bowel cancer treatment of Ono Pharmaceutical Korea, and Pfizer's Lorviqua, were set. The HIRA (Director Kim Sun-min) today (12th) released the "Result of Review on benefit Standards for Drugs for Cancer Patients" reviewed by t
- Policy
- Daewoong tx are being developed for skin side effects
- by Lee, Tak-Sun Jan 18, 2022 06:06am
- Daewoong Pharmaceutical has started to develop a treatment for skin side effects caused by anticancer drugs. This treatment is known as a stem cell-based drug. The MFDS approved the phase 2 clinical trial plan for DWP708 submitted by Daewoong Pharmaceutical on the 17th. This clinical trial is conducted to explore the efficacy of DwP1708 an
- Policy
- Discord in system hinder coverage of rare disease drugs
- by Lee, Jeong-Hwan Jan 18, 2022 06:06am
- A suggestion has been raised that it is irrational for a drug that received an orphan drug designation to be ineligible for insurance benefits that were set for rare disease treatments to improve patient accessibility because its disease was not designated as a rare disease or set for special exemption of calculation. In other words, the trea
- Policy
- The gov will push for a refund on losses from suspension
- by Kim, Jung-Ju Jan 18, 2022 06:05am
- The government will push for a revision of the law that will put a brake on the suspension of execution filed by pharmaceutical companies in drug price lawsuits. If companies fight against the government over drug prices and finally win the case, the insurer will refund the drug cost equivalent to the loss so far. This is a revision of the legal
- Company
- Pharma exports exceed ₩9 trillion in 2021
- by Kim, Jin-Gu Jan 18, 2022 06:05am
- Pharmaceutical exports in Korea have exceeded &8361;9 trillion last year. With exports increasing around biopharmaceuticals, sales of the drugs increased over 2.2 times over the past 2 years. Imports have also reached record-high, influenced by the increased import of Pfizer and Moderna’s COVID-19 vaccines. On the other hand, the export of
- Opinion
- [Desk] The task of reimbursement for ultra-high-priced drugs
- by Kim, Jung-Ju Jan 18, 2022 06:05am
- The evaluation of the adequacy of the super-high-priced Kymriah's benefit is approaching insurance coverage. This is because the agenda to expand the health insurance standards for Kymriah, a treatment for acute lymphocytic leukemia and lymphoma CAR-T, and Keytruda, a non-small cell lung cancer immuno-cancer drug, passed side by side at the H
- Company
- First PIK3CA-targeting ‘Piqray’ lands in general hospitals
- by Eo, Yun-Ho Jan 17, 2022 05:51am
- ‘Piqray,’ the first targeted anticancer drug that targets the PIK3CA gene, may now be prescribed at general hospitals. According to industry sources, Novartis Korea’s ‘Piqray (alpelisib)’ passed the drug committee (DC) reviews of 2 of the ‘Big 5’ hospitals in Korea - the Seoul Samsung Medical Center, and Seoul Asan Medical Center.
- Policy
- A plan to expand patient access to Kymriah should be passed
- by Kim, Jung-Ju Jan 17, 2022 05:51am
- While the agenda for the health insurance registration of the ultra-high-priced innovative drug Kymriah and the expansion of immuno-cancer drug Keytruda benefits have been in full swing since the afternoon of today (13th), patient groups gathered in front of the Kukje Electronic Center's Smart Work Center. Patient maximum co-payment of
- Company
- Shortening the completion of Samsung Biologics plant 4
- by Kim, Jin-Gu Jan 17, 2022 05:51am
- Samsung Biologics announced that it will start partial operation within this year in connection with its fourth plant currently under construction. It also introduced that it has already won orders for consignment production from three global pharmaceutical companies. John Lim, CEO of Samsung BioLogics, made the remarks at a press conference
- Company
- Korean companies' progress in developing COVID-19 treatment
- by Moon, sung-ho Jan 17, 2022 05:51am
- News of the decision to introduce Pfizer’s oral COVID-19 treatment ‘Paxlovid’ in Korea from earlier this year has increased interest in the progress made in the development of oral COVID-19 treatments at Korean pharmaceutical companies. The interest in when the homegrown oral COVID-19 treatments will be commercialized was triggered by the