- Company
- New triple combo ‘Enerzair’ for asthma lands in 'Big 5'
- by Eo, Yun-Ho Jan 6, 2022 06:09am
- ‘Enerzair Breezhaler,’ a three-in-one triple combination therapy for asthma, can now be prescribed at general hospitals in Korea. According to industry sources, Novartis Korea’s triple combination drug ‘Enerzair Breezhaler (mometasone·indacaterol·glycopyrronium)’ that contains ICS (inhaled corticosteroids) · LABA (beta2-agonist)
- Product
- Lee’s pledge to reimburse hair loss drug creates buzz
- by Jung, Heung-Jun Jan 6, 2022 06:09am
- The news that presidential candidate Lee Jae-Myung's is considering reimbursement of hair loss treatments as his election pledge is drawing attention for days. However, as many unresolved issues including creating empathy on its need, budgetary projections, and review of beneficiaries remain, it is unclear whether this may be practically
- Policy
- There were no applications for Ultomiris·Soliris benefits
- by Lee, Hye-Kyung Jan 6, 2022 06:09am
- In November last year, it was confirmed that there was no pre-application for Ultomiris and Soliris benefits for new patients with paroxysmal night hemoglobin (PNH). There were only two applications for pre-approval of Soliris and one application for re-examination approval for new patients with atypical hemolytic uremic syndrome (aHUS), all
- Policy
- Companies obtain generic for exclusivity in proportion
- by Lee, Tak-Sun Jan 6, 2022 06:08am
- Among pharmaceutical companies that use generic for exclusive in the licensed patent linkage system that took effect in 2015, it was found that companies with large sales are more likely to obtain generic for exclusivity. As a result, it was suggested that small and medium-sized pharmaceutical companies need more support such as consulting.
- Policy
- Request for non-reimbursed use of Pahtension in Loop's pts
- by Lee, Hye-Kyung Jan 6, 2022 06:08am
- Application for Pahtension 20mg was rejected for non-reimbursed patients with systemic erythema lupus accompanied by immunosuppressants, vasculitis that does not improve even with antibiotics, and peripheral ulcers. The HIRA is receiving applications in advance for use exceeding the MFDS' permission to prevent the use of drugs that lack medic
- Policy
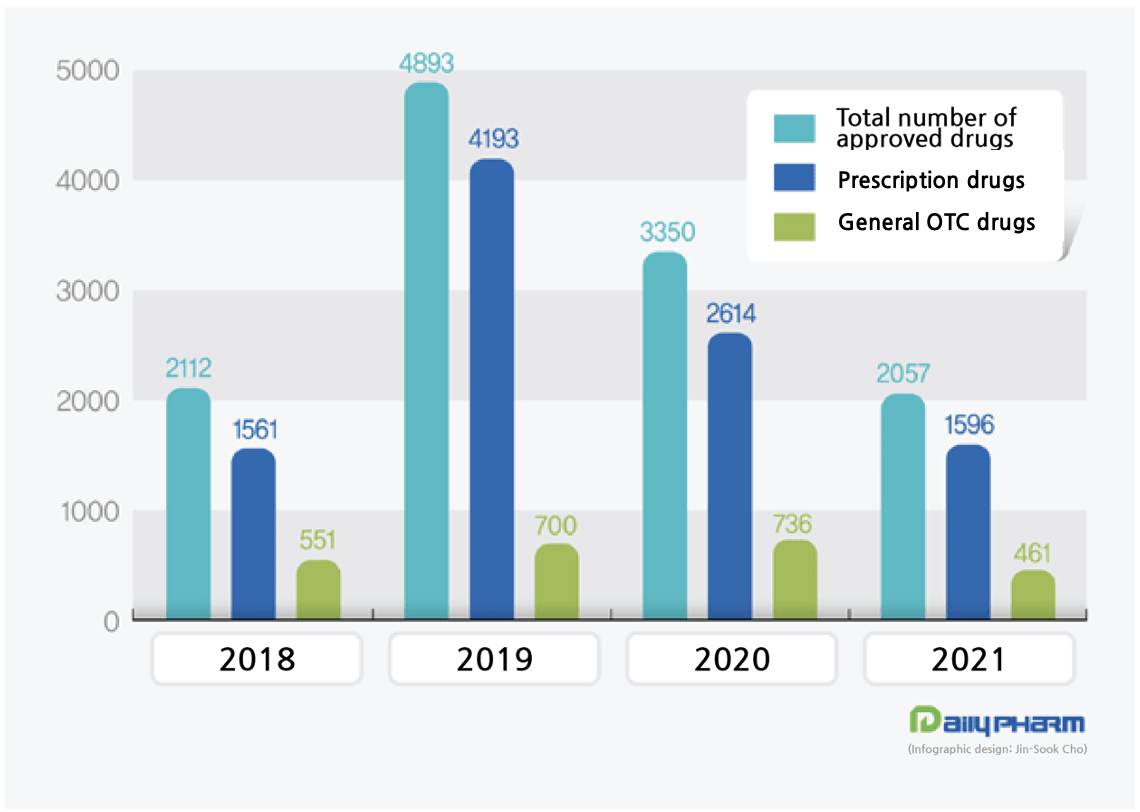
- ‘Lowest-ever’ number of drugs approved in 4 years
- by Lee, Tak-Sun Jan 5, 2022 05:59am
- The year 2021 is likely to be remembered as the year of reduced marketing authorizations for drugs. This was greatly influenced by the changes in the market and the new regulations that were introduced last year. In particular, the pricing penalty imposed on indirect bioequivalence tested drugs and the restrictions set on consigned bioequivalenc
- InterView
- There shouldn't be any patients who can't receive new drugs
- by Eo, Yun-Ho Jan 5, 2022 05:59am
- Global pharmaceutical companies, which can be said to be the mainstay of the supply of new drugs. The KRPIA, which represents these companies, is also raising expectations in 2022. Multinational pharmaceutical companies' attention is more focused on the "appropriate value of new drugs" than ever before. With the advent of the so-called "high-
- Opinion
- [Reporter’s View]Year of regulations, principle vs practice
- by Kim, Jin-Gu Jan 5, 2022 05:57am
- As in every other year, regulations imposed on the pharmaceutical and biopharmaceutical industry are again expected to be further reinforced this year. In particular, GMP-related regulations will be strengthened even further. Starting this month, the government may impose punitive fines on the arbitrary manufacture of pharmaceuticals by c
- Company
- Exports of the biohealth industry surpassed 19 trillion won
- by Jan 5, 2022 05:57am
- Exports of the domestic biohealth industry hit a record high last year due to soaring demand for biosimilars, COVID-19 diagnostic kits and medical devices. Exports in related sectors reached $16.2 billion last year, exceeding $15 billion for the first time. It is expected that strong performance will continue this year due to the release of new
- Policy
- Combination drugs of PPI+ antacid have also been released
- by Lee, Tak-Sun Jan 5, 2022 05:57am
- In the anti- ulcer drug market, combination drugs of PPI+ antacids have been released. This time, it is a drug that combines Rabeprazole and sodium bicarbonate. The PPI+ antacids, which started with Chong Kun Dang's Eso Duo (Esomeprazole Magnesium Trihydrate+Sodium Bicarbonate), continues to develop new products. The MFDS approved Youngjin