- Company
- Latecomer JAKi ‘Rinvoq’ now has the most indications
- by Oct 8, 2021 05:56am
- The latecomer JAK inhibitor, Abbvie’s ‘Rinvoq,’ is seeking to overturn the market landscape by greatly expanding its indications. Whether the drug can break the two-way lead battle between Xeljanz and Olumiant, is gaining attention. According to the Ministry of Food and Drug Safety, Abbvie’s ‘Rinvoq (upadacitinib)’ received addition
- Policy
- Expanding the submission of opinions reflecting permission
- by Lee, Tak-Sun Oct 7, 2021 05:54am
- Opportunities for submitting opinions from industries will be expanded when reflecting permits based on the results of the reexamination. A pre-announcement procedure is added to the previous opinion inquiry. The MFDS announced that since the 27th of last month, the procedure for reflecting permits based on the results of the reexamination
- Policy
- ’With Covid’ scheme unclear with over 5,000 cases expected
- by Lee, Jeong-Hwan Oct 7, 2021 05:53am
- Concerns have been raised that Korea will be unable to adopt the ‘With Corona’ scheme due to the public’s distrust in vaccinations, the government’s non-acceptance of casualties of adverse reactions from vaccines, and the surge in daily COVID-19 cases, etc. Based on the mathematical model that took into account current incidence, the tran
- Policy
- Nexviazyme has been applied for domestic permission
- by Lee, Tak-Sun Oct 7, 2021 05:53am
- Sanofi plans to release a new Pompe's disease treatment in Korea. It is known that Nexviazyme, which was approved by the U.S. FDA in August, recently applied for permission from the MFDS. According to the MFDS on the 1st, Sanofi Aventis Korea submitted a report on the results of clinical trials by Nexviazyme (Avalglucosidase alfa-ngpt) and
- Policy
- Introduction of pre-registration is difficult
- by Kim, Jung-Ju Oct 7, 2021 05:53am
- The government said it is difficult due to concerns over weakening NHIS' drug price negotiation power, while various fields are proposing the introduction of a system that is first registered and evaluated later for access to treatments for severe rare and intractable diseases. Regarding referring to Korean drug prices such as China, the gove
- Company
- Roche and Lilly to launch RET targeted cancer drugs in Korea
- by Eo, Yun-Ho Oct 6, 2021 06:06am
- Two types of RET targeted anticancer therapies are concurrently seeking domestic entry. According to industry sources, Roche Korea's Gavreto (pralsetinib)’ and Lilly Korea ‘Retevmo (selpercatinib)’ are under review for domestic approval. Both are anticancer drugs that target RET (Rearranged during transfection) gene fusions. The d
- Company
- Evrysdi (PO) is differentiated due to its low price
- by Oct 6, 2021 06:06am
- Roche's "Evrysdi (Risdiplam)," the second treatment for spinal muscular atrophy (SMA) in Korea, signaled its launch after about a year of approval. With the differentiation of the only PO drug and low prices, fierce competition in the SMA market was predicted. "Evrysdi is the first oral drug among SMA treatments and is applicable to patien
- Policy
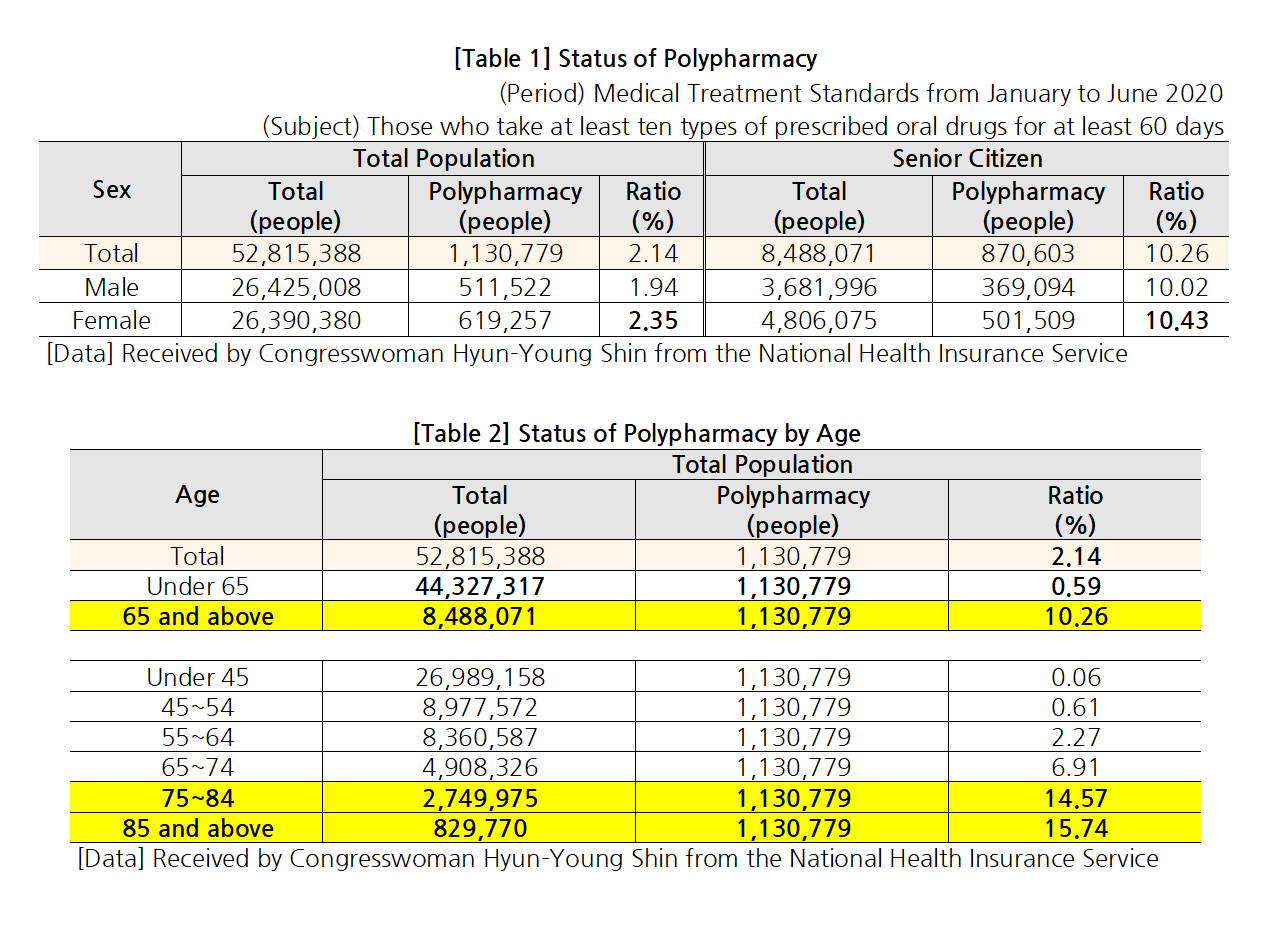
- “Elderly polypharmacy rate Korea 70% vs OECD countries 48%"
- by Lee, Jeong-Hwan Oct 6, 2021 06:06am
- Statistics showed that a high rate - 70.2% - of older adults (aged 75 or over) in Korea chronically take ‘more than 5 drugs for over 3 months.’ The average of 7 OECD countries other than Korea that submitted the same data was only 48%. In other words, concerns over the current polypharmacy status of elderly patients in Korea have been r
- Policy
- The patient died due to the delay in Kymriah benefits
- by Lee, Jeong-Hwan Oct 6, 2021 06:06am
- Korea leukemia patients organization launched a one-man protest, urging the first C-ART treatment Kymriah (Tisagencleucel)'s fast track. The Korea Leukemia Association held a press conference in front of the National Human Rights Commission of Korea at 10 a.m. on the 1st and announced that it submitted a petition to the National Human Rig
- Company
- Yoo Byung-Jae took office as the new president of Novartis
- by Oct 6, 2021 06:05am
- Yoo Byung-Jae took office on the 1st as the new president of Novartis Korea. New President Yoo is an expert who has accumulated organizational management and management skills in the global healthcare industry for 15 years. Until recently, he served as president of North Asia, including South Korea, Taiwan, and Hong Kong, at Johnson & Joh