- Company
- Pfizer’s 2nd JAK inhibitor ‘Cibinqo’ to soon enter Korea
- by Eo, Yun-Ho Sep 30, 2021 05:57am
- Pfizer is planning to introduce a new JAK inhibitor after ‘Xeljanz’ to Korea. However, the new drug will target the ‘atopic dermatitis’ treatment market. According to industry sources, Pfizer Korea has recently submitted an application requesting marketing authorization for its Cibinqo (abrocitinib). The approval is expected to be mad
- Company
- It does not affect sales of Rivaroxaban 2.5mg
- by Kim, Jin-Gu Sep 30, 2021 05:57am
- Hanmi dismissed the possibility of legal disputes over the patent registration of the original company ahead of the launch of Xarelto's generic. The company said that the scope of validity of patents registered late does not affect Hanmi's new drug to be released. In addition, Hanmi emphasizes that it did not take issue when it sent certi
- Policy
- Pts can participate in Phase III tx for terminal cancer
- by Lee, Tak-Sun Sep 30, 2021 05:57am
- The MFDS announced on the 29th that it has revised and distributed the "Guidelines for applying rapid screening of medicines" so that even early cancer patients can participate in clinical trials, considering the difficulty of recruiting large-scale phase 3 clinical trials for terminal cancer patients. However, even in the early stages of the
- Policy
- 24 new drugs receive ₩177.9 billion benefit this year
- by Kim, Jung-Ju Sep 30, 2021 05:56am
- A total of 24 new drugs were newly listed or expanded reimbursement standards by this month, improving patient accessibility. Among new drugs that were already listed, reimbursement standards for 4 products were extended, improving coverage. Although only 10,000 patients in Korea will benefiting from the extension, the result could be interp
- Company
- The cumulative sales of Biktarvy amounted to ₩60 billion
- by Sep 30, 2021 05:56am
- Gilead Science's Biktarvy dominated the AIDS tx market around the world, including Korea, two years after its launch. Biktarvy consists of Bictegravir Sodium, Emtricitabine, and Tenofovir Alafenamide Fumarate as Gilead's HIV treatment. Compared to conventional treatments, the effectiveness and safety are improved, the expression rate of resi
- Company
- SK C&C-GC Pharma, establish a big data platform for healthca
- by Chon, Seung-Hyun Sep 30, 2021 05:56am
- SK C&C announced on the 27th that it will carry out a project with GC Pharma Holdings to build a comprehensive healthcare big data analysis platform based on artificial functions (AI). During the project period, the two companies analyze and map various standard medical data based on the "cloud-type digital platform" and conduct AI convergence a
- Company
- Generic for Xarelto is about to be released.
- by Kim, Jin-Gu Sep 30, 2021 05:56am
- Bayer's new oral anticoagulant (NOAC) Xarelto (Rivaroxaban)' generic is imminent to be released. In particular, Hanmi, which received generic exclusivity, plans to release Xarelto 2.5mg exclusively, and Hanmi has a patent dispute with Bayer, drawing keen attention to the future response of the two companies. According to the pharmaceutical in
- Policy
- Will a treatment for resistant hypertension be released?
- by Lee, Tak-Sun Sep 29, 2021 05:54am
- Attention is focused on whether a treatment for resistant hypertension that cannot be controlled with existing drugs, that is in the final stages of its clinical trial, will succeed in commercialization. The drug is firibastat, that is developed by Quantum Genomics. Dong Wha Pharmaceuticals owns exclusive commercialization rights to supp
- Policy
- DUR check for more than 28 days of Zolpidem combined Rx
- by Lee, Hye-Kyung Sep 29, 2021 05:54am
- In the future, 49 drug ingredients, such as "Zolpidem," that should be careful during the administration period, will also be checked for drugs that exceed the maximum administration period by adding up the number of prescription days. The HIRA announced that it will apply "improvement of DUR inspection standards" to 345 items (213 reimubrsed dr
- Policy
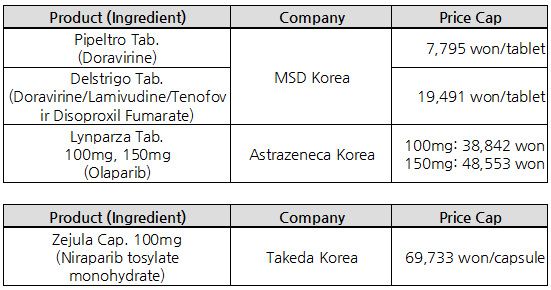
- Delstrigo listed at ₩19,491 … Zejula’s price cut 6%
- by Kim, Jung-Ju Sep 29, 2021 05:53am
- MSD Korea’s fixed-dose combination product Delstrigo (doravirine·lamivudine·tenofovir disoproxil fumarate) will be listed with insurance benefit next month, at &8361;19,491. Also, reimbursement for Takeda Korea’s Zejula cap. 100mg (niraparib) has been extended to cover monotherapy for ovarian cancer, upon which the price was cut by 6% and w