- Policy
- Azelnidipine was approved in Korea
- by Lee, Tak-Sun Sep 17, 2021 05:56am
- Azelnidipine, which was approved in 2003 in Japan as a calcium channel blocker (CCB), was also approved in Korea. There was no previously approved finished product. The MFDS approved Azelnidipine 8mg of Introbiopharma on the 14th. Azelnidipine is a treatment for hypertension, a drug administered oral after breakfast once a day. Previous
- InterView
- "A tenure professor’s calling is in developing a new drug"
- by Sep 17, 2021 05:56am
- On August 24th, the Hemato-Oncology Department of the Eijeongbu Eulji Medical Center was busy preparing for its new occupant. Professor Dongwook Kim (60), who looked new to his office, was busy discussing matters with various visitors including the hospital employees. Although the center had opened less than 6 months ago, its Hematol-Oncology De
- Policy
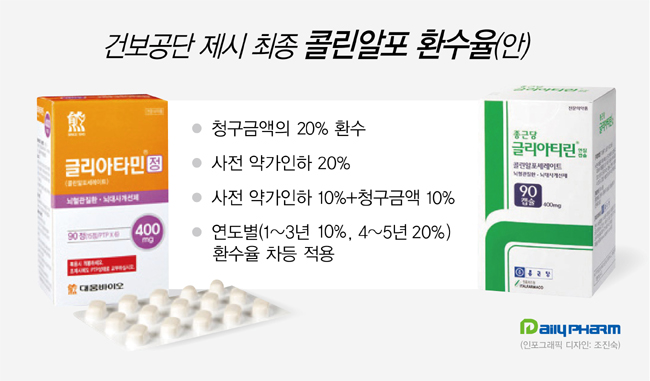
- 58 companies agree on the redemption of α- GPC benefits
- by Lee, Hye-Kyung Sep 17, 2021 05:56am
- Negotiations on the conditional return of benefits for clinical reevaluation of "Choline alfoscerate," a brain function improving agent that has been going on for about nine months, will end today (15th). The final result is that all 58 pharmaceutical companies with 123 Choline alfoscerate agreed to "return 20% of their health insurance claim
- Company
- Blockbuster anticancer drug series 4 - Avastin
- by Sep 17, 2021 05:55am
- "By blocking blood vessels generated to proliferate cancer, cancer is starved to death.There is a drug that realizes the theory of a professor at Harvard University in the U.S., who caused a "sensation" in the 1970s. Avastin, a Vascal Endothermic Growth Factor (VEGF) inhibitor, which is also considered a good partner for immuno-cancer drugs.
- Company
- JAK inhibitors may fall to 2nd-line due to safety concerns
- by Nho, Byung Chul Sep 16, 2021 05:59am
- With the health authorities seriously considering changing the reimbursement standards for Janus kinases (JAK) inhibitors which have recently been caught up in controversy over its safety issues, what the results will be is gaining industry-wide attention. According to industry sources, the Ministry of Health and Welfare (MOHW) and the He
- Company
- Nocdurna can be prescribed at general hospitals
- by Eo, Yun-Ho Sep 16, 2021 05:59am
- iNight urination treatment "Nocdurna" has been settled on the prescription ticket of a general hospital. According to related industries, Nocdurna (Desmopressin), co-sold by Ferring and Chong Kun Dang, passed DC, drug committee of Big 5 General Hospital such as SNUH, SMC, Seoul St. Mary's Hospital, and except for Sinchon Severance Hospital.
- Policy
- Will Jeil Pharm gain a hold over the varenicline market?
- by Lee, Tak-Sun Sep 16, 2021 05:59am
- Following the government’s measures to reduce impurities in smoking cessation drugs that contain varenicline, Jeil Pharamceutcial’s products, which satisfy the set standards, is expected to gain a hold over the smoking cessation treatment market for the time being. The Ministry of Food and Drug Safety has allowed the lot release of vare
- Policy
- Drug substances with impurities are collected voluntarily
- by Lee, Tak-Sun Sep 16, 2021 05:59am
- The recovery of antihypertensive drugs exceeding impurities is expanding. Following the Drug product, Drug substance began to recover. The MFDS announced on the 14th that it will voluntarily retrieve some of Kukjeon and Pharmacostech's Irbesartan's Drug Substance. Kukjeon and Pharmacostech start collecting four manufacturing number products e
- Company
- Idiance reveals the results of Venadaparib
- by Kim, Jin-Gu Sep 16, 2021 05:59am
- Idience, a new drug development company of Ildong Holdings, announced on the 14th that it will announce the results of phase 1b clinical trials of the targeted anticancer drug Venadaparib (IDX-1197) at the ESMO conference to be held from the 16th to the 21st. Venadaparib is a new drug candidate for targeted anticancer drugs based on precision
- Policy
- 299 items were voluntarily withdrawn
- by Lee, Hye-Kyung Sep 15, 2021 06:12am
- The NHIS's self-evaluation came out that the "National Health Insurance Medical Care Benefit Standards" revised on October 8 last year prevented the registration at will. At the Korea Special Press Association briefing held on the 14th, Lee Sang-soo, executive director of insurance benefit, said, "As soon as the drug benefit list was regis