- Opinion
- [Reporter's view] A manual has been established
- by Lee, Tak-Sun Sep 13, 2021 05:55am
- The recovery of hypertension treatments containing excess impurity Sartans is significant in that it has established a new manual for drug recovery. All items were banned and recovered, making it difficult for manufacturing, sellers, and medical institutions to do additional work due to collection. However, since only excess Sartan items
- Policy
- Hanmi pushes for Champix follow-on despite hardships
- by Lee, Tak-Sun Sep 13, 2021 05:55am
- One pharmaceutical company is determined to develop a follow-on of the smoking cessation drug, ‘Champix’ (Pfizer, varenicline tartrate), and it is Hanmi Pharmaceuticals. Hanmi Pharmaceuticals had succeeded in the early release of its drug as a salt-modified drug, but had lost the patent challenge failed to enter the market. After the
- Policy
- Voluntary recovery of 73 drugs with excess impurities
- by Lee, Tak-Sun Sep 13, 2021 05:55am
- 73 items from about 36 companies with blood pressure exceeding impurities will be recovered. Patients can also be exchanged for normal products at pharmacies. However, the MFDS explained that concerns about human harm are very low, and that it is not a situation to stop taking them arbitrarily. It announced on the 9th that some of the drugs c
- Company
- Jardiance marks new milestone in heart failure treatment
- by Whang, byung-woo Sep 13, 2021 05:55am
- With the SGLT-2 inhibitor, Jardiance (empagliflozin), proving its efficacy in heart failure with preserved ejection fraction (HFpEF), on how it will affect the domestic prescription market for heart failure is gaining attention. As another SGLT-2 inhibitor, Forxiga (dapagliflozin), was the first to receive approval for heart failure with redu
- Policy
- Financial savings should be given for new domestic drugs
- by Lee, Jeong-Hwan Sep 10, 2021 05:58am
- The domestic pharmaceutical industry argued that new domestic development drugs, which contributed to improving the financial soundness of health insurance through steady R&D investment, should be excluded from the list of "The Price-Volume Agreement (PVA)" or introduced a system that limits the number of applications. Even after the registra
- Company
- Key 3 mRNA vaccine techs are not Moderna's nor Pfizer's
- by Kim, Jin-Gu Sep 10, 2021 05:58am
- The three key technologies required for developing mRNA vaccines were revealed. These are technologies related to antigen optimization, mRNA syntehsis·modification, and manufacture of lipid nanoparticles (LNP) that correspond to steps 1, 2, and 4 of the 5-step vaccine manufacturing process. The explanation was that a biopharmaceutical
- Policy
- The evaluation criteria's determination for reimbursed drugs
- by Lee, Hye-Kyung Sep 10, 2021 05:58am
- If the administration cost is lower than that of alternative drugs, it is possible to apply for adjustment to raise the upper limit of the drug. However, it should be the case where the number of companies in the formulation with the same administration route or component is one. The HIRA recently guided the pharmaceutical industry to answer que
- Policy
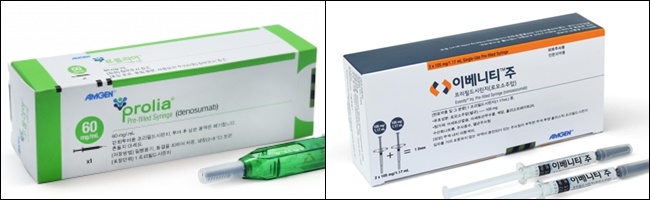
- Will it be possible to expand the benefit of Prolia?
- by Lee, Jeong-Hwan Sep 10, 2021 05:58am
- Attention is focusing on whether the criticism that biopharmaceuticals that treat osteoporosis by preventing bone absorption or activating bone formation are not fully enjoyed due to restrictions on Korea's benefit standards can be resolved. This is directly related to Amgen's Prolia (Denosumab) and Evenity (Romosumab) access to patients, as
- Company
- Medytox’s American dream falters with returned botox rights
- by Kim, Jin-Gu Sep 9, 2021 05:55am
- Medytox’s plans to overcome the license revocation in Korea by entering the US market, have come to a halt with AbbVie, which had been conducting global trials for the new botulinum toxin candidate 'MT10109L,’ returning its rights to Medytox. ◆A technology export agreement worth up to &8361;400 billion was returned in 8 years
- Policy
- A bill to revise some of the Jeju Special Law
- by Lee, Jeong-Hwan Sep 9, 2021 05:55am
- The bill banning the opening of for-profit hospitals and pharmacies in Jeju is aimed at preventing controversy over the Greenland International Medical Center by deleting special provisions on the opening of foreign medical institutions and foreign-only pharmacies. The plan aims to eliminate controversy that could cause confusion in the do