- Policy
- No. of refunded RSA drugs become 23 with addition of Onivyde
- by Lee, Hye-Kyung Aug 9, 2021 06:00am
- With the new addition of Servier Korea's pancreatic cancer treatment 'Onivyde (irinotecan liposome)' that was approved for reimbursement, a total of 23 drugs are now being covered under the Risk-sharing Agreement (RSA) scheme. Patients who take RSA drugs need to bear the full cost of the drug, then request refunds to the applicable pharmaceut
- Policy
- It will spend ₩2.2 trillion to become a vaccine power
- by Lee, Jeong-Hwan Aug 9, 2021 06:00am
- President Moon Jae-in has announced that he would leap forward into a 'global vaccine production, the Big Five' by 2025. It also announced plans to select vaccines as one of the 'three major national strategic technologies' along with semiconductors and batteries and invest &8361;2.2 trillion over the next five years. On the 5th, Presi
- Policy
- Attention is focused on licensing domestic vaccines
- by Lee, Jeong-Hwan Aug 9, 2021 06:00am
- As the supply price of COVID vaccines, which relies entirely on imported products, is confirmed to increase next year's supply price in Korea, it is necessary to develop the vaccine within this year. Attention is focusing on the development status of Korean pharmaceutical bio companies, which are about to undergo phase 3 clinical trials, w
- Company
- Sales of 'Stillen' have declined in two years
- by Chon, Seung-Hyun Aug 8, 2021 06:50pm
- Sales of gastritis treatments containing Artemisia Herb as an active ingredient have decreased. Sales had increased due to reflective profits from withdrawal of Ranitidine, but fell in two years. It is analyzed that Artemisia Herb's sales have decreased as the prescription market has shrunk due to the prolonged COVID-19 outbreak and PPI drugs ha
- Company
- Celltrion's developing next-generation mRNA vaccine platform
- by Lee, Seok-Jun Aug 8, 2021 06:49pm
- Celltrion has started developing next-generation mRNA vaccine platform with TriLink Biotechnology in the U.S. According to the company on the 4th, TriLink is a contract development and manufacturing organization (CDMO) based on the mRNA platform located in San Diego, U.S. It has its own Vactor and Gen3 CleanCap, which are essential for the
- Company
- Pfizer seeks generation change in the ALK market
- by Aug 8, 2021 06:48pm
- Pfizer, which developed the first ALK-targeted treatment, has released a third-generation new product. A new option has emerged in the ALK targeted treatment market, where subsequent drugs were not appropriate. Third-generation drugs are also expected to compete with second-generation products as a primary treatment. Pfizer was approved by
- Policy
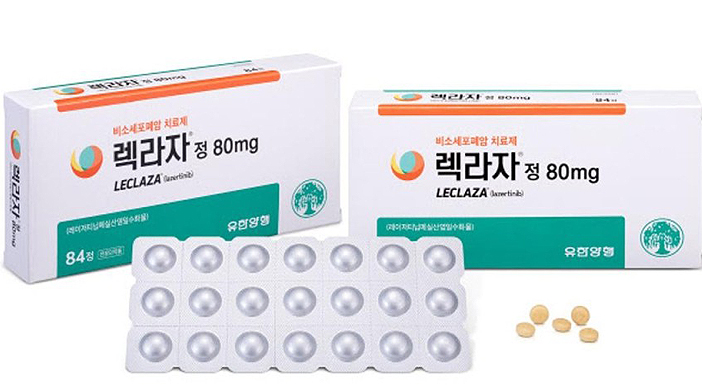
- PBAC results on Leclaza that was listed in 165 days show...
- by Lee, Hye-Kyung Aug 6, 2021 06:05am
- ‘Leclaza tab. (lazertinib mesylate monohydrate),' the 31st novel drug to be developed in Korea that received approval for insurance benefit in only 165 days after its approval, was reviewed as a 'new treatment alternative for non-small cell lung cancer patients' in the evaluation process for assessing the reasonableness of the medical care ben
- InterView
- “Olumiant provides rapid AD symptom improvement"
- by jung, sae-im Aug 6, 2021 06:04am
- The introduction of JAK inhibitors in the field of moderate-to-severe atopic dermatitis (AD) treatment was like a welcome rain in the drought of treatment options in AD. In 2018, the approval of the biologic agent ‘Dupixent’ transformed the AD treatment paradigm, however, many patients still feel an unmet need existed due to the limited re
- Policy
- Last year, the drug trade surplus was achieved
- by Lee, Tak-Sun Aug 5, 2021 08:47pm
- Domestic medicine achieved its trade surplus for the first time last year. This is because biosimilar led the export. The MFDS announced on the 1st that it analyzed the production, export, and import performance of drugs, quasi-drugs in 2020. Drugs achieved the trade surplus (&8361;1.394 trillion won) for the first time since 1998, when the MFD
- Company
- Generic share in the Tamsulosin market has surpassed 60%
- by Kim, Jin-Gu Aug 5, 2021 08:46pm
- In the Tamsulosin prostate hypertrophy treatment market worth &8361;170 billion per year, Rx amount of generics increased by 10% compared to last year. During the same period, the original decreased by 10%, with generic accounting for more than 60% of the total market. According to UBIST, a pharmaceutical market research institute on the