- Company
- Keytruda's sales in Q1 of the year are ₩9 trillion
- by Aug 5, 2021 08:46pm
- MSD's Keytruda (Pembrolizumab)'s global quarterly sales surpassed $4 billion for the first time, ranking first in immuno-cancer drugs. According to related industries on the 2nd, the total global performance of five Checkpoint inhibitors (Keytruda, Opdivo, Tecentriq, Imfinzi, and Yervoy) in the first half of this year was $15.558 billion,
- Company
- Sales of PPI antiulcer agents rise 30% in 2 years
- by An, Kyung-Jin Aug 5, 2021 06:03am
- The ranitidine impurity issue gave wings to the rising prescription of proton pump inhibitors (PPIs). The share of PPI prescriptions in the antiulcer agent market grew 30% in only 2 years after the drugs containing ranitidine &8211; which used to occupy the largest share of the H2 receptor antagonists - were pulled from the market. Hanmi’s inc
- Product
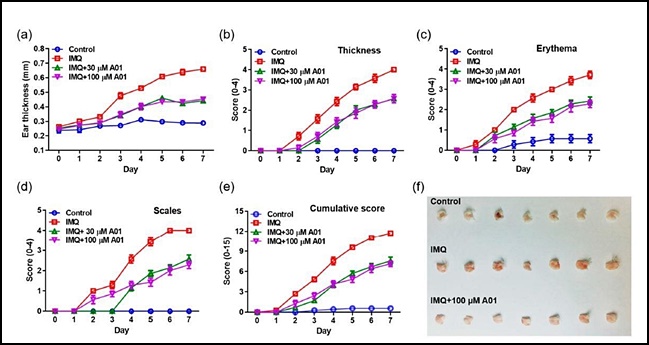
- "Inhibiting ANO1 activity has effect on treating psoriasis"
- by Lee, Jeong-Hwan Aug 5, 2021 06:02am
- A study result has shown that inhibiting the activity of the Anoctamin-1 (ANO1) protein may be effective in treating psoriasis, an intractable skin disorder. The results are expected to aid further research in developing ANO1 inhibitors for the treatment of intractable diseases such as psoriasis and cancer. On the 4th, the joint research t
- Company
- Restylane Kysse has been approved in Korea
- by jung, sae-im Aug 5, 2021 12:41am
- Galderma Korea announced on the 2nd that Hyaluronic acid filler Restylane Kysse was approved by the MFDS on July 26 for the purpose of temporarily expanding the volume of lips for adults aged 21 or older. Restylane Kysse, which received Korea's first permission for lip fillers, was developed by Galderma as Optical Balanced Technology( OBT)
- Company
- Roche’s Polivy can be prescribed at general hospitals
- by Eo, Yun-Ho Aug 5, 2021 12:40am
- The new lymphoma drug Polivy can be prescribed at general hospitals. According to related industries, ADC that combine with conventional BR therapy (Bendamustine/ Rituximab) treatments for diffuse large B-cell lymphoma (DLBCL) that have poor non-responsive prognosis of Roche. However, Polivy is still a non-reimbursed drug. Roche applied i
- Policy
- No prior approval req. filed for Soliris, 47 for Ultomiris
- by Lee, Hye-Kyung Aug 4, 2021 06:01am
- ‘Ultomiris (ravulizumab),’ a follow-on drug of ‘Soliris (eculizumab)’ that was approved for reimbursement from June, has taken away all new prescriptions for the treatment of paroxysmal nocturnal hemoglobinuria (PNH) from Soliris. Reimbursement for Ultomiris, like Soliris, needs to be authorized in advance through a prior authorization ap
- Policy
- Will changes be made to Hemlibra’s reimbursement standards?
- by Lee, Jeong-Hwan Aug 4, 2021 06:01am
- As the Anti-Corruption & Civil Rights Commission (ACRC) in addition to the National Assembly raised the need to ease the reimbursement standards for the hemophilia treatment Hemlibra (emicizumab), the growing sense of urgency on the Ministry of Health and Welfare (MOHW) and the Health Insurance Review and Assessment Service (HIRA)’s behalf
- Policy
- Daewoong has added a line-up of osteoporosis treatments PO
- by Lee, Tak-Sun Aug 3, 2021 08:27pm
- Daewoong's Evimax 45mg was approved on July 29. It is generic for Raloxifene HCl 45mg. The original for Raloxifene HCl is Evista from Alvogen Korea. Evista contains Raloxifene HCl 60 mg. Raloxifene HCl 45mg was first developed by Yuhan. In September 2019, Yuhan was granted 'Raboni 45mg', a product containing Raloxifene HCl 45mg. The comp
- Policy
- The number one treatment for mild COVID is Kaletra
- by Kim, Jung-Ju Aug 3, 2021 08:24pm
- The number one drug used to treat mild COVID in the first half of this year was AbbVie's Kaletra, which amounted to &8361;596 million. The total amount of drugs spent on the entire treatment, including severe cases, amounted to &8361;12.1 billion. Among the data on "COVID-19 Treatment Expenditure in the first half of the year," the "Status
- Company
- Will regulations on ERP be established?
- by Aug 3, 2021 08:24pm
- Lee Nak-yeon, a presidential candidate for the Democratic Party of Korea, mentioned the need to draw up regulations on the Early Retention Program (ERP) of foreign-invested companies, including foreign pharmaceutical companies. Attention is focusing on whether frequent ERP regulations in the pharmaceutical industry will be possible. He mad