- Policy
- Pfizer aims to commercialize PF-07321332 by the end of 2021
- by Lee, Tak-Sun Aug 3, 2021 08:09pm
- On the 29th, the MFDS approved 3 multi-national clinical plans for "PF-07321332," a candidate for COVID-19 oral treatment applied by Pfizer. The first clinical trial was the oral administration of PF-07321332 to prevent postmortem infections in adults who had contact with people with COVID-19, and the validity and safety of Ritonavir's tw
- Company
- Samsung launches Humira Biosimilar ‘Adalloce’ in Korea
- by Eo, Yun-Ho Aug 3, 2021 07:04am
- Domestic supply of the domestic biosimilar of ‘Humira’ has begun in full scale. According to industry sources, Adalloce, a biosimilar of the TNF- alpha blocker Humira(adalimumab)’s that was developed by Samsung Bioepis and sold by Yuhan Corporation in Korea, passed the Drug Committees (DCs) of 20 medical institutions including the Big
- Policy
- Peramivir has earned its generic exclusivity
- by Lee, Tak-Sun Aug 2, 2021 08:41pm
- JW Life Science is the first known Peramivir drug to win a generic for exclusivity. It is known to have been applied by JW Life Science's premium technology. Premix preparation is an injection that does not need to be diluted with saline water and is convenient to use. However, GC Pharma and Chong Kun Dang were also allowed to impose prem
- Company
- Beijing Hanmi's 2Q operating profit increased by 50%
- by Chon, Seung-Hyun Aug 2, 2021 08:37pm
- Hanmi Pharmaceutical's second-quarter performance improved. In the domestic market, self-developed new drug products have done well. Sales of Beijing Hanmi have more than doubled despite the sluggishness caused by COVID-19. Hanmi announced on the 29th that its operating profit in the second quarter increased 49.6% year-on-year to &8361;15.9
- Policy
- Reimbursement criteria for Cosentyx and Taltz inj. expanded
- by Kim, Jung-Ju Aug 2, 2021 06:06am
- Secukinumab injections such as Novartis Korea’s Cosentyx injection and Ixekizumab injections such as Lilly Korea’s Taltz prefilled syringe injection will be covered as first-line biologics for active and progressive psoriatic arthritis refractory to DMARDs. Also, the monitoring cycle of liver and electrolyte levels in patients for orall
- Company
- ACRC "Hemlibra's reimb. standards need to be reexamined"
- by Kim, Jin-Gu Aug 2, 2021 06:05am
- On the 30th, the Anti-Corruption & Civil Rights Commission (ACRC) has expressed the opinion that the reimbursement standards for the hemophilia treatment Hemlibra should be reexamined. In addition, the ACRC forwarded their official statement to the Ministry of Health and Welfare (MOHW) and the Health Insurance Review and Assessment Service (
- Company
- Daewoong recovers with record 2Q sales of ₩289.7 billi
- by An, Kyung-Jin Jul 30, 2021 05:48am
- Daewoong has continued its good performance for 2 consecutive quarters. By overcoming the heavy blows it took from the impurity issue in ranitidine and the lawsuits from its botulinum toxin strain dispute, the company was able to raise its quarterly sales to the highest level despite the COVID-19 crisis. Daewoong Pharmaceuticals has poste
- Company
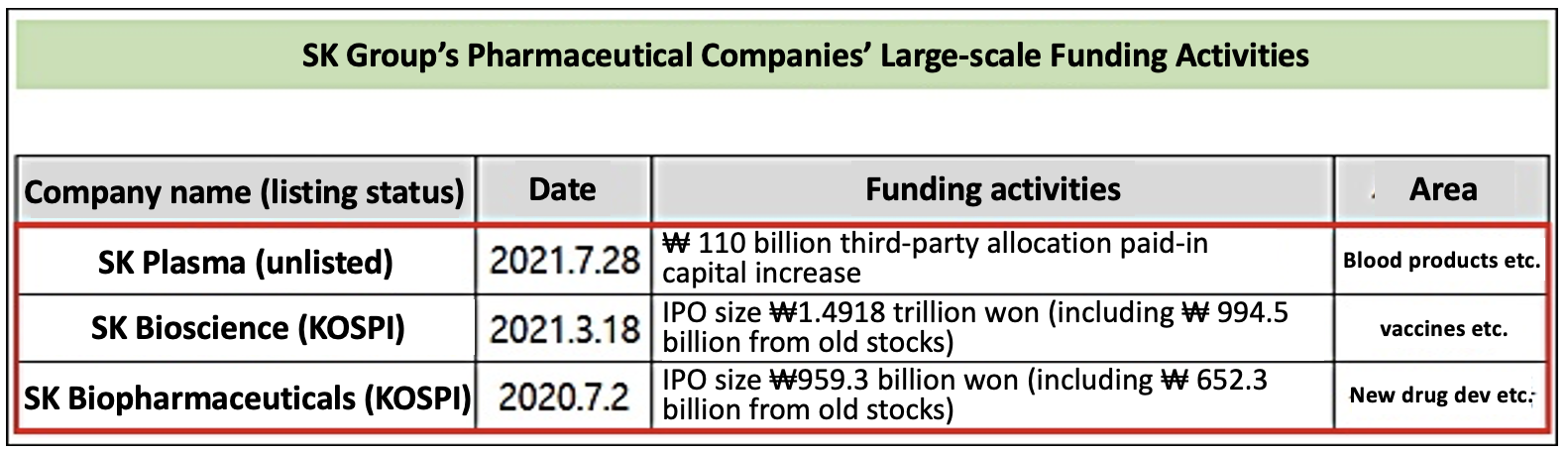
- SK Group's Pharma companies make successive success
- by Lee, Seok-Jun Jul 30, 2021 05:48am
- SK Group’s pharmaceutical companies have successively succeeded in raising large-scale funds. The analysis is that being able to invest in independent corporations differentiated by business characteristics attracted market investment. SK Group’s pharmaceutical companies, which all use the SK name, are operated in different areas by SK or S
- Company
- Emgality and Ajovy were also approved
- by Kim, Jin-Gu Jul 30, 2021 05:47am
- Following Lilly's Emgality (Galcanezumab), Teva's Ajovy (Fremanezumab) were released in Korea as a new medicine for migraine prevention. Attention is focusing on whether CGRP (calcitonin gene-related peptide) drugs, which have entered the domestic migraine treatment market without suitable treatments, will become popular. Aimovig is not
- Company
- Chong Kun Dang applied for Lucentis biosimilar
- by Chon, Seung-Hyun Jul 30, 2021 05:47am
- Chung Kun Dang announced on the 28th that it has applied to the MFDS for permission for the item of the macular denaturation drug CKD-701. CKD-701(Ranibizumab) is a biosimilar product from Lucentis. Lucentis, sold by Roche and Novartis, is a drug used to treat ophthalmic diseases such as macular degenerative diabetes and macular edema. Lucent