- Policy
- Quality issues alarmed the pharmaceutical industry
- by Jun 22, 2021 05:50am
- Recent cases of GMP violations by Korean pharmaceutical companies have served as an opportunity to guard against risks throughout the industry. In addition, there are opinions that it should be improved in the wake of this incident. Until now, actual changes in standards for GMP have been sluggish according to global level. Only a few compani
- Policy
- Closing the opinion gap important for the generic '1+3 bill'
- by Lee, Jeong-Hwan Jun 21, 2021 05:51am
- With the bill limiting the participating generic makers to three consignees per consignor when conducting joint biological equivalence tests or clinical tests awaiting review by the National Assembly’s Legislation and Judiciary Committee., whether the opinion gap between the large pharmaceutical companies and small- and medium-sized pharm
- Company
- Competition between PCSK9 Inhibitors has just begun
- by Eo, Yun-Ho Jun 21, 2021 05:51am
- The competition for prescription of PCSK 9 inhibitors began more than four years after the domestic approval. Sanofi-Aventis' Allepatadine (Olopatadine) was listed on the 7th. It was approved in January 2017. This was the first time in South Korea that PCSK's 9th suppression system was introduced. Later in April of the same year, Amgen's R
- Policy
- Gov will proactively improve system for severe psoriasis
- by Kim, Jung-Ju Jun 21, 2021 05:50am
- With a year left before the re-registration of special exemption of insurance calculation for severe psoriasis, the government, payer, and patient group gathered to discuss improvement. The issue discussed was that despite reimbursement approved for severe psoriasis drugs, patients are not being properly covered as the eligibility standards for
- Policy
- Yuhan's Raboni-D has been licensed
- by Lee, Tak-Sun Jun 21, 2021 05:50am
- Yuhan, which had a high dependence on sales for imported drugs, has recently been speeding up with the commercialization of new drugs such as Lazertinib and IMD. In particular, Yuhan refrains from entrusting or entrusting developing products and is building market competitiveness with its own products. The MFDS approved "Raboni-D," Yuhan's
- Product
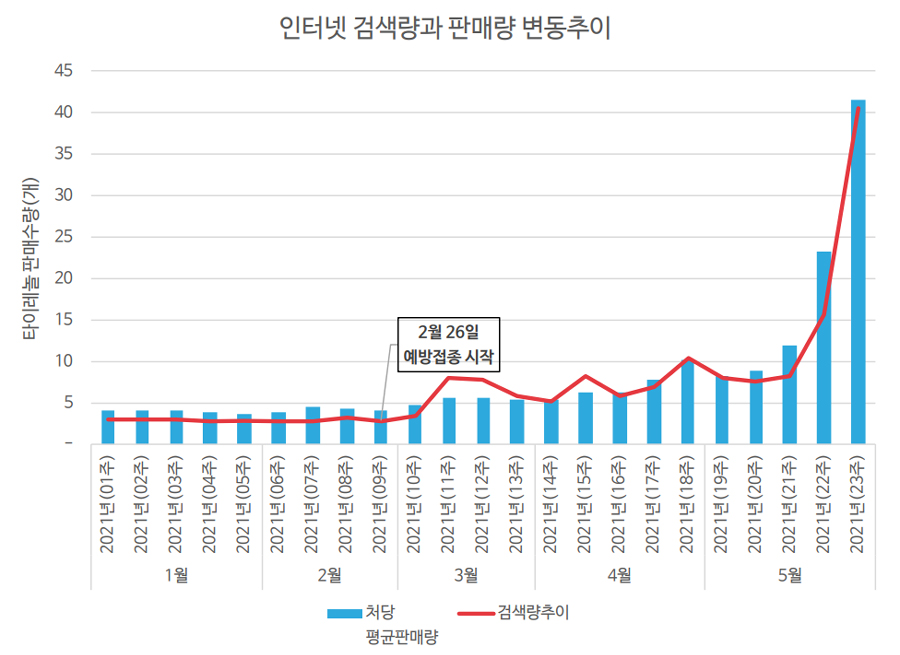
- Tylenol sales increased 10 times to peak in May
- by Jun 21, 2021 05:50am
- Pharmacies' difficulties have reached their peak due to the surging demand for Tylenol, which is said to be "more precious than COVID-19 vaccine." This is because Tylenol has become so precious that it is rarely found in pharmacies. Then how popular was Tylenol? Analysis of POS data from 185 pharmacies showed that sales of Tylenol 500 m
- Company
- JAK inhibitor ‘Olumiant’ seeks reimb for atopic dermatitis
- by Eo, Yun-Ho Jun 18, 2021 05:54am
- The JAK inhibitor ‘Olumiant’ is seeking extended reimbursement benefit in atopic dermatitis. According to industry sources, Lilly Korea has submitted an application for the reimbursement of Olumiant (baricitinib) in ‘the treatment of adult patients with moderate to severe atopic dermatitis who are candidates for systemic therapy’. The
- Policy
- An exception to the 1+3 Bill for IMD
- by Lee, Jeong-Hwan Jun 18, 2021 05:54am
- A letter from a pharmaceutical representative to the National Assembly affected the process of the National Assembly's Health and Welfare Committee's handling of generics, drug for data-based re-evaluation "1+3 bill." It was reflected in the revised schedule when a representative of company A sent a petition to 24 members of the National A
- Policy
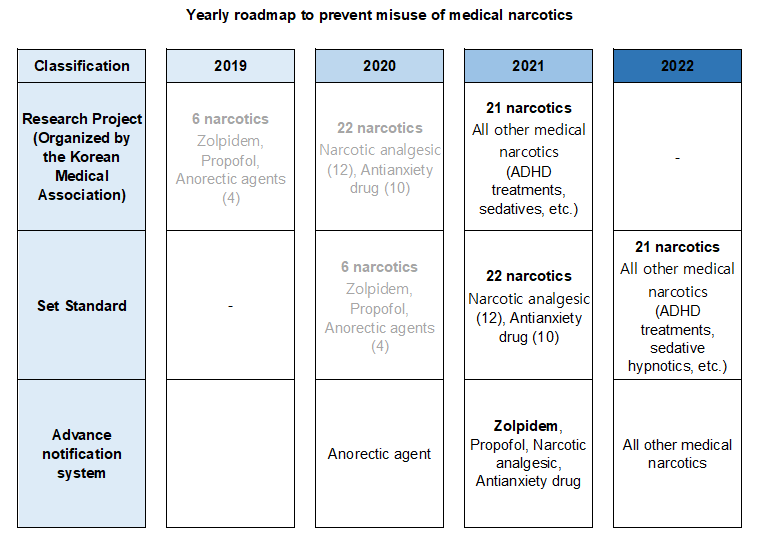
- 559 doctors warned for wrongful prescription of Zolpidem
- by Lee, Tak-Sun Jun 18, 2021 05:53am
- After analyzing the prescription information on the Narcotics Information Management System (NIMS), the Ministry of Food and Drug Safety announced its decision to issue written ‘warnings’ to 559 doctors that have continuously prescribed or used Zolpidem beyond the safe use standards to prevent abuse and promote proper use of the medical narcot
- Policy
- “The 1+3 bill” was passed by the Welfare Committee
- by Lee, Jeong-Hwan Jun 18, 2021 05:53am
- The 1+3 bill passed a plenary session of the National Assembly's Health and Welfare Committee on the morning of the 16th and will be reviewed by the legislation and judiciary committee. The resolution reflected the revision of the supplementary provision, which excludes the report from the MFDS within a month from the enforcement date of t