- Policy
- AZ vaccine, the first inoculation in Korea from the 26th
- by Kim, Jung-Ju Feb 18, 2021 06:24am
- The domestic schedule for the COVID-19 vaccination treatment made by AstraZeneca has been confirmed. The government plans to fully implement vaccinations from the 26th, but plans to accumulate and determine additional clinical information for those over 65 years old people. Inoculation of AZ vaccine to the elderly over 65 is virtually wit
- Company
- Does Propecia cause depression?
- by Eo, Yun-Ho Feb 17, 2021 05:40am
- It has already been reflected in the package insert such as Korea and Europe, and has not been directly proven to have a causal relationship. There was another controversy that the hair loss drug Propecia causes depression. Reuters reported on the 3rd (local time) of the lawsuit filed in the Brooklyn Federal Court of New York in connection
- Policy
- AstraZeneca Korea earns Consignment Business Authorization
- by Lee, Tak-Sun Feb 17, 2021 05:40am
- AstraZeneca Korea earned the seventh ‘Pharmaceutical Consignment Manufacturing Business Authorization’ in South Korea due to the COVID-19 vaccine manufactured by SK Bioscience. South Korea’s Ministry of Food and Drug Safety (MFDS) reported AstraZeneca Korea has been authorized for a pharmaceutical consignment manufacturing business o
- Policy
- Hanmi’s salt-modifying drug for Galvus, re-licensed
- by Lee, Tak-Sun Feb 17, 2021 05:39am
- Hanmi, which failed to enter the market for Vildagliptin through so-called split indications, has obtained item approval again. The MFDS approved Vildagle Tab 50mg (Vildagliptin HCl) by Hanmi on the 10th. It was licensed in January of last year, and Vildagle Tab 50mg, which was voluntarily withdrawn in July of that year. The withdrawn item
- Company
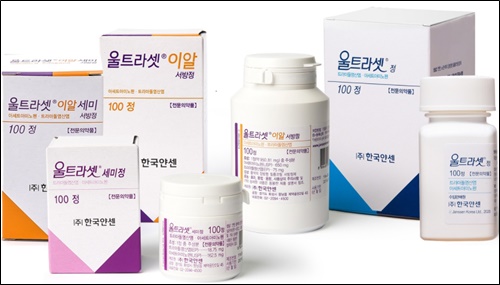
- SK Chemicals sells Janssen’s Ultracet
- by Lee, Seok-Jun Feb 17, 2021 05:39am
- SK Chemicals announced on the 15th that it has signed a domestic sales contract with Janssen Korea for the anti-pain inflammatory drug Ultracet (Acetaminophen/ Trimadol HCl). According to this agreement, SK Chemicals is in charge of Ultracet distribution ,domestic marketing and sales. Janssen Korea will be in charge of production. There
- Opinion
- [Reporter’s View] KDCA’s dilemma on using AZ vaccine
- by Kim, Jin-Gu Feb 17, 2021 05:39am
- The last call is to be made by the hand of Korea Disease Control and Prevention Agency (KDCA). On Feb. 10, South Korea’s Ministry of Food and Drug Safety (MFDS) finalized the authorization on AstraZeneca’s COVID-19 vaccine. The ministry included a warning for inoculating an age group over 65 that ‘the vaccination should be carefully consi
- Policy
- Novavax vaccine needed as AZ vaccine in 65 and up postponed
- by Lee, Tak-Sun Feb 16, 2021 06:08am
- The South Korean government seems to be considering on purchasing more doses of other COVID-19 vaccine options as the government has decided to hold off on inoculating the age group over 65 with AstraZeneca’s vaccine. Originally, the 65-and-up age group was supposed to be vaccinated within the first quarter, but it has been pushed to t
- InterView
- Takeda's evolution continues
- by Eo, Yun-Ho Feb 16, 2021 06:08am
- It is difficult to stop what is going well. Even more so if it is a company's business. Takeda sold its diabetes and over-the-counter (OTC) business to Celltrion, a domestic company, last year. Takeda's Actos is a TZD-family drug that persisted in the Avandia outbreak, while Whituben and Albothyl are famous OTCs that everyone knows, mean
- Policy
- COVID-19 vaccine lot release to be authorized this week
- by Lee, Tak-Sun Feb 16, 2021 06:07am
- As a COVID-19 vaccine was authorized for the first time in South Korea, the lot release for AstraZeneca’s vaccine is expected to be granted this week. Although South Korea’s Ministry of Food and Drug Safety (MFDS) did not restrict inoculation on the age group over 65, the Korea Advisory Committee on Immunization Practices (KACIP) assoc
- Policy
- Fosamax 10mg·Pletaal OD Tab has been withdrawn
- by Lee, Tak-Sun Feb 16, 2021 06:07am
- MSD Korea's osteoporosis treatment Fosamax, and Otsuka Pharmaceutical's antiplatelet drug Pletaal OD Tab will be withdrawn from the Korean market due to poor performance. According to the MFDS on the 9th, as of the 8th, MSD Korea's Fosamax 10mg (Alendronic acid), Otsuka's Pletaal OD Tab 100mg, Pletaal OD Tab 50mg, and Pletaal Powder 20% (