- Company
- Celltrion's CT-T43 was approved for Phase III clinical trial
- by Kim, Jin-Gu Jan 13, 2021 06:10am
- Celltrion announced on the 8th that it has received approval for the Phase III clinical trial plan of Stelara (Ustekinumab)'s biosimilar. Janssen's autoimmune disease treatment Stelara is a mechanism that inhibits interleukin (IL)-12·23, and has indications for psoriasis, Crohn's disease, and ulcerative colitis. It is known that global s
- Company
- What domestic pharmaceuticals JP Morgan paid attention to?
- by Moon, sung-ho Jan 12, 2021 06:22am
- More than 20 domestic pharmaceutical and bio companies are invited to the world's largest pharmaceutical and bio industry event, annual J.P. Morgan Healthcare Conference. According to the pharmaceutical industry on the 8th, more than 20 domestic pharmaceutical and bio companies, including Samsung Biologics and Hanmi, will participate in the J.P.
- Policy
- 9 Atozet generic companies to join CKD via consignment
- by Lee, Tak-Sun Jan 12, 2021 06:22am
- Nine pharmaceutical companies, initially preparing to launch a generic version of a dyslipidemia treatment Atozet (atorvastatin plus ezetimibe), joined a group of manufacturers to produce Chong Kun Dang’s evidence-submitting drug as CMO. Instead of generics applying for the health authority approval from this month, the companies seem
- Company
- MSD received administrative disposition for packaging
- by Whang, hyung-woo Jan 12, 2021 06:19am
- MSD Korea has been subject to administrative disposition for violating the provisions of supplying small packages. However, it was confirmed that they were looking for a way through the exception. Considering the characteristics of taking 2 tablets a day, it applied for relief saying that it had to admit an exception to the packaging of 60 ta
- Policy
- Breaking down MOHW 2021 action plan for NHI Master Plan
- by Kim, Jung-Ju Jan 12, 2021 06:19am
- The drug reimbursement benefit would be improved throughout chronic disease treatment this year, centering hepatitis B and C virus treatment and antidiabetic combination drugs. The already listed drug reimbursement reevaluation would be enforced from the latter half of the year, when the subjects are decided in the first half of the year. Mo
- Company
- HK inno.N wins big in huge shift of global vaccine licenses
- by Kim, Jin-Gu Jan 11, 2021 06:11am
- Started from late last year, the massive shift in South Korean market sales rights over global vaccines has come to an end. Global companies like MSD, GSK and Sanofi Pasteur, and South Korean companies like GC Pharma, SK Bioscience, HK inno.N, Yuhan Corporation and Handok were involved in the mass migration of the businesses. The industry se
- Policy
- 22 generics for Atozet by Chong Kun Dang were approved
- by Lee, Tak-Sun Jan 11, 2021 06:10am
- The products of 22 companies for hyperlipidemia (Ezetimibe/Atorvastatin) consigned by Chong Kun Dang were approved on the 8th. As a result, 20 generisc within the same ingredient were approved, and the next drug price application for the same drug product was reduced to the lowest price. This is because of the stepped drug price system tha
- Policy
- Plasma treatment was completed in Phase II clinical trial
- by Lee, Tak-Sun Jan 11, 2021 06:10am
- The government confirmed that the patient administration of the clinical II trial of a domestically developed blood system drug has been completed. As in the case of Celltrion's antibody treatment, the application for Conditional Marketing Authorization (CMA) is imminent. Kwon Jun-wook, the 2nd vice-president of the Central Disease Control
- Policy
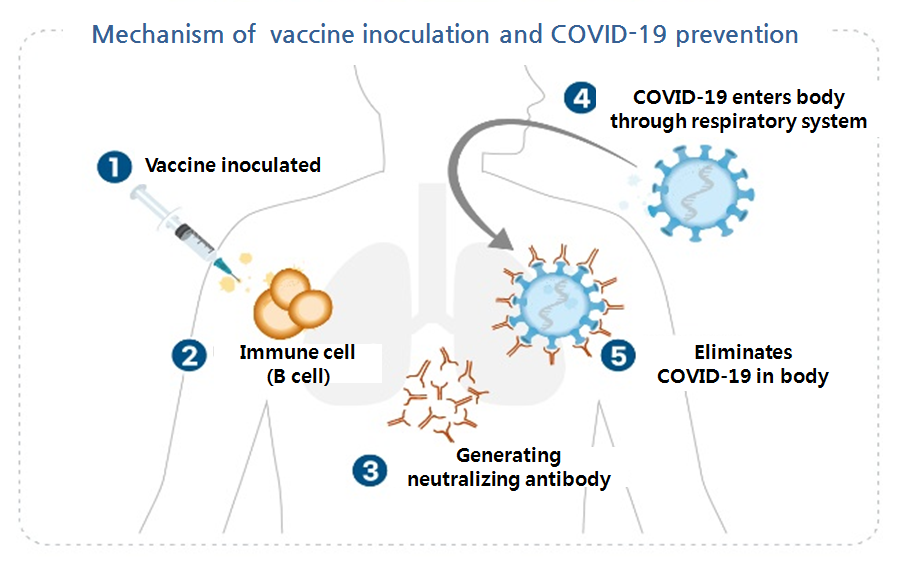
- Adenovirus for AZ vaccine, Sinopharm uses inactivated virus
- by Lee, Tak-Sun Jan 11, 2021 06:10am
- Diverse types of COVID-19 vaccines are currently in development. Pfizer and Moderna’s vaccine candidates are based on RNA, when AstraZeneca’s is a virus vector and Korean-based SK Bioscience’ is a recombinant vaccine. These types of vaccine have their respective strengths and weaknesses. Some types have been commercialized already, but m
- Company
- Allergan Korea names Kim Sook-hyun as new CEO
- by Jan 11, 2021 06:10am
- Allergan Korea Aesthetic-AbbVie Company said it has selected Kim Sook-hyun as the new CEO as of Jan. 1 She is a global healthcare professional with 22 years of experience in Korea, Asia, and headquarters receiving an MBA at Kelley School of Business after graduating from industrial pharmacy at Seoul National University. She joined Abbo