- Company
- Immunotherapy Keytruda faces Cancer Committee once again
- by Eo, Yun-Ho Jun 5, 2020 06:09am
- An immunotherapy Keytruda would be standing before the Cancer Deliberation Committee once again. On June 3, Keytruda (pembrolizumab) would be deliberated by Health Insurance Review and Assessment Service (HIRA) Cancer Deliberation Committee again, although the committee deferred the decision in a meeting finally held on Apr. 29 after COV
- Policy
- Permission is increasing prior to the end of PMS
- by Lee, Tak-Sun Jun 4, 2020 10:41pm
- Even before the end of PMS (Post marketing surveillance), a lot of new items have been approved through a contract production contract with the original company. Since PMS system in Korea is also provided with the data protection function of the original drug, it is possible to apply for a license for the same ingredient after PMS expires.
- Company
- Janssen revs up 2 new global trials on lazertinib
- by An, Kyung-Jin Jun 4, 2020 06:14am
- Janssen’s novel anticancer treatment lazertinib licensed out from Yuhan is initiating a new global clinical trial. Prior to a global phase 2 trial, the multinational company is accelerating the investigational drug’s commercialization process with other phase 1 trials. According to a clinical trial registration website ‘clinicaltrials
- Policy
- Labeling will be promoted on product packaging
- by Lee, Tak-Sun Jun 4, 2020 06:14am
- In the future, it is expected that pharmaceutical companies carrying out bioequivalence tests will be separately labeled on generic drug packaging. In addition, policy initiatives are being undertaken to strengthen the global competitiveness of generic drugs, such as finding ways to disclose generic companies on a manufactory basis. The MF
- Opinion
- [Reporter’s view] Pharmacists are tired of impurities
- by Kim JiEun Jun 4, 2020 06:13am
- Another task has recently been added to the pharmacy. From Valsartan, Ranitidine to Metformin. This is because pharmacies have to clean up with repeated impurities situation. The Metformin situation was stopped by the sale of some items, so there was not much confusion than the previous Valsartan or Ranitidine events. However, due to the p
- Company
- 3 out of 10 executives, worries about poor performance
- by Chon, Seung-Hyun Jun 3, 2020 06:40am
- The executives working at the pharmaceutical company pointed out the poor performance after COVID-19 outbreak. Most people pointed out that the company should reduce its performance targets to minimize damage to COVID-19. On the 21st anniversary of its founding, Dailypharm conducted a survey of “Post COVID-19 Crisis Response Strategy”
- Company
- Cancer Committee to review 3 signature drugs by BMS-Celgene
- by Eo, Yun-Ho Jun 3, 2020 06:25am
- On June 3, the Cancer Deliberation Committee is to review granting coverage on key products by the combined company of Bristol Myers Squibb (BMS) and Celgene. Celgene’s multiple myeloma pipelines&8212;Revlimid (lenalidomide) and Pomalyst (Pomalidomide)&8212;and BMS’ immunotherapy Opdivo (nivolumab) plus Yervoy (ipilimumab) combinatio
- Policy
- The MFDS designated Mobocertinib as an orphan drug
- by Lee, Tak-Sun Jun 3, 2020 06:25am
- The MFDS (Minister Eui-kyung Lee) announced that Mobocertinib was newly designated as an orphan drug, and it announced on June 1 that target diseases were added for three types, such as Ipilimumab, designated as an orphan drug. An orphan drug is a drug that is used for the purpose of diagnosing or treating a rare disease, a drug that has
- Company
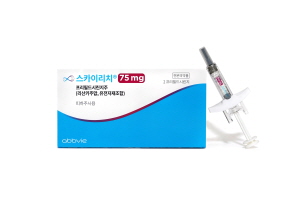
- AbbVie’s psoriasis drug Skyrizi NHI listed from June 1
- by Eo, Yun-Ho Jun 3, 2020 06:25am
- Another interleukin inhibitor has been listed for National Health Insurance (NHI) reimbursement. AbbVie Korea announced the Korean health authority is providing healthcare reimbursement on severe psoriasis treating interleukin-23 (IL-23) inhibitor Skyrizi (risankizumab) from June 1. The coverage would be granted to patients with chro
- Policy
- Handok takes over Eisai’s Aricept license from Daewoong
- by Lee, Tak-Sun Jun 3, 2020 06:25am
- The Korean market license for the dementia treatment market leader Aricept (donepezil) has been transferred from Daewoong Pharmaceutical to Handok. Except for a few items, Handok is to manufacture the treatment in Korea. Daewoong Pharmaceutical has been importing active ingredient from a Japanese-based pharmaceutical company Eisai that