- Company
- K-pharma trials show greenlight overcoming bile duct cancer
- by Son, Hyung Min Apr 4, 2025 05:58am
- South Korean and the overseas pharmaceutical industry's candidate products that are targeted anticancer agents for bile duct cancer (cholangiocarcinoma) are showing results of effectiveness, indicating the potential for commercialization possibilities. Korean and overseas pharmaceutical companies, including Handok's US partner Compass Therapeuti
- Company
- Obesity drug craze affects global pharma subsidiaries in KOR
- by Son, Hyung Min Apr 4, 2025 05:58am
- Whether or not a new obesity drug in the glucagon-like peptide (GLP-1) family has been launched has had a significant impact on the performance of Novo Nordisk and Lilly Korea. Last year, Novo Nordisk’s sales increased by 63% upon the launch of Wegovy in the domestic market. In the case of Lilly Korea, sales decreased slightly due to the delay
- Company
- New drug 'Niktimvo' for cGVHD receives ODD in Korea
- by Eo, Yun-Ho Apr 4, 2025 05:58am
- 'Niktimvo,' a new drug for the treatment of chronic graft-versus-host disease (cGVHD), has been designated as an orphan drug in South Korea. The Ministry of Food and Drug Safety (MFDS) announced this on the notifications of Orphan Drug Designation (ODD). Niktimvo (axatilimab)'s basis for ODD indication is for the treatment of 'adults an
- Policy
- COVID-19 Vaccine Damage Compensation Act passes NA
- by Lee, Jeong-Hwan Apr 4, 2025 05:58am
- A special law that compensates and supports patients who have suffered damage after COVID-19 vaccination was passed at the plenary session of the National Assembly on the afternoon of the 2nd. Also, a partial amendment to the Framework Act on Health and Medical Services, which includes the establishment of a Health and Medical Manforce Pl
- Company
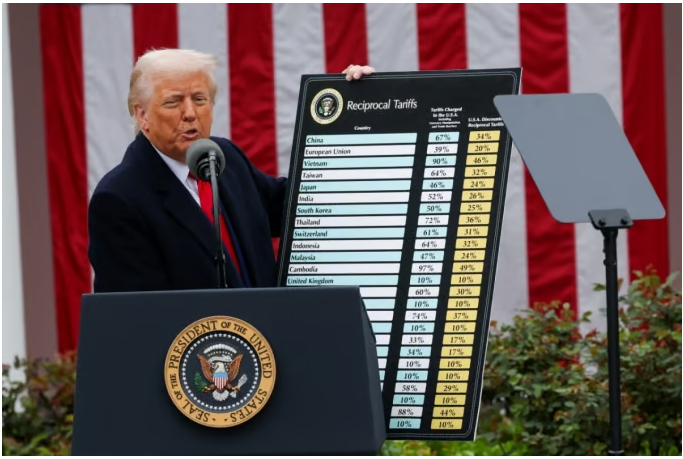
- US postpones 'Drug Tariffs', but industry eyes the situation
- by Kim, Jin-Gu Apr 4, 2025 05:57am
- The US Trump administration announced reciprocal tariffs against the entire world, but “postponed” the application of tariffs on pharmaceuticals, because it is “considering separate industry-specific tariffs” on semiconductors, key minerals, and pharmaceuticals. The domestic pharmaceutical and bio industry is relieved for now but is
- Company
- Qarziba may be prescribed in general hospitals in KOR
- by Eo, Yun-Ho Apr 3, 2025 05:55am
- Qarziba, an immunotherapy drug that targets neuroblastoma, may now be prescribed at general hospitals in Korea. According to industry sources, Recordati Korea's immunotherapy drug for high-risk, recurrent, and refractory neuroblastoma, Qarziba Inj (dinutuximab beta), has passed the drug committees (DCs) of the ‘Big 5 general hospitals’
- Company
- "COVID-19 differs from influenza…vaccination essential"
- by Whang, byung-woo Apr 3, 2025 05:55am
- "COVID-19 can lead to 'long COVID,' and its progression tends to be more severe among the elderly and immunocompromised individuals. It is crucial to emphasize COVID-19 prevention as the saying goes, 'an ounce of prevention is worth a pound of cure.' After the announcement of the endemic in 2023, COVID-19 has repeatedly mutated, thus regarded
- Opinion
- [Reporter's View] Preparations for US pharmaceutical tariff
- by Kim, Jin-Gu Apr 3, 2025 05:55am
- US President Donald Trump designated April 2 (local time) as 'America's Day of Liberation.' The day the US will announce reciprocal tariff rates for various countries. US reports expect details on tariff-affected countries, rates, and applicable product categories to be disclosed as early as the evening of the 1st. The specific tariff rat
- Company
- J&J MedTech launches PFA Varipulse platform
- by Whang, byung-woo Apr 3, 2025 05:54am
- Johnson & Johnson MedTech announced on the 1st that it will launch the Varipulse platform, an arrhythmia treatment solution that uses three-dimensional pulsed field ablation (PFA), in Korea. As the most common arrhythmia in the world, Atrial fibrillation (AFib) is a condition in which the atria beat irregularly and rapidly. In particu
- Company
- ‘Leclaza combo will become the sole standard of care’
- by Son, Hyung Min Apr 3, 2025 05:54am
- &160;“The Leclaza + Rybrevant combination therapy has shown a clear improvement in survival compared to Tagrisso monotherapy in the MARIPOSA trial. I am confident that this combination therapy will become the sole standard treatment for EGFR-positive non-small cell lung cancer in the future.” On the 31st, Professor Byoung-chul Cho of t