- Company
- Daxas' generic by Sama succeeded in patenting
- by Kim, Jin-Gu Apr 2, 2020 06:26am
- Sama Pharm succeeded in avoiding the patent for Daxas (Roflumilast), a treatment for chronic obstructive respiratory disease (COPD). Sama Pharm's success in avoiding this patent attracts attention because large companies have failed in succession. The IPT took the side of a generic company in a trial to confirm the passive scope of the
- Policy
- Marketing approval for major Ranitidines have been renewed
- by Lee, Tak-Sun Apr 2, 2020 06:26am
- The approval of Ranitidine formulations, such as Albis, Curan, and Zantac, which have been banned from the end of September last year due to the detection of carcinogenic substances NDMA, has been renewed. Accordingly, permits will remain in effect until March 31, 2025. It is noted whether sales can be resumed during this period. According
- Policy
- NHIS to act on growing number of administrative litigations
- by Lee, Hye-Kyung Apr 2, 2020 06:26am
- National Health Insurance Service (NHIS) is setting down administrative litigation response strategy to handle pharmaceutical companies’ taking legal action against drug pricing reduction. NHIS has recently started a cosigned research on ‘Drug Pricing Reduction Related Litigation Case Study and Response Plan.’ The research would be
- Policy
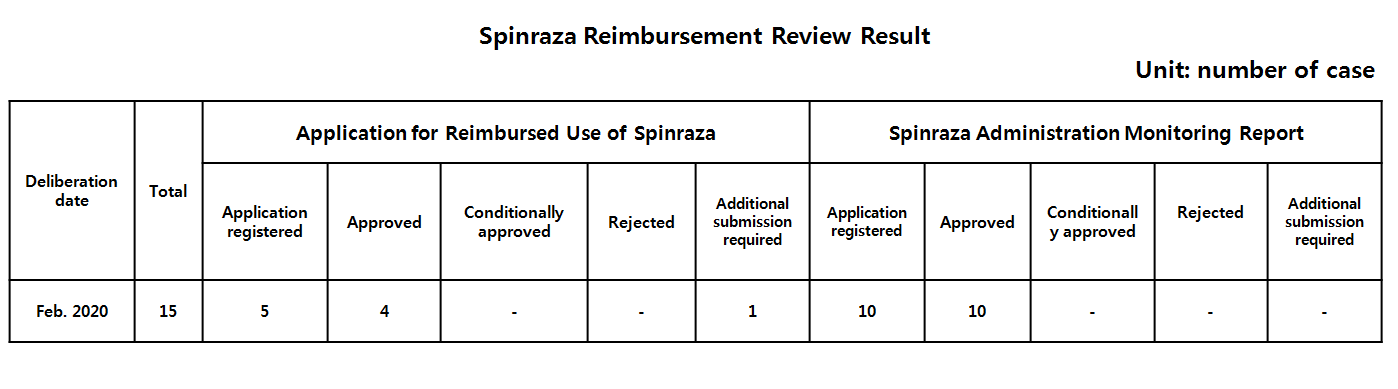
- HIRA clears 4 out of 5 Spinraza reimbursement pre-approvals
- by Lee, Hye-Kyung Apr 2, 2020 06:26am
- Korean health authority has passed four out of five preliminary applications submitted last month to treat spinal muscular atropy (SMA) with reimbursed use of Spinraza. Even the one denied case would likely to be cleared, if the applicant submits additional evidential data of patient’s onset symptoms of SMA. In every four months, Sp
- Company
- Hemlibra's indications are expanded
- by Nho, Byung Chul Apr 2, 2020 06:25am
- As a global new drug, indications & dosage standards for Hemlibra, a hemophilia preventive drug, have been expanded. JW Pharmaceutical said on the 31st, that Hemlibra (Emicizumab), which dramatically improves the convenience of administration, is additionally approved by the MFDS as a routine preventive therapy for non-antibody patients.
- Policy
- Co-pay rate of advanced hospitals for cold patients to 80%
- by Lee. Chang jin Apr 1, 2020 06:21am
- As soon as the plan to reorganize the medical delivery system, which has shown a lull in the event of COVID-19, is expected to be presented to the Health Insurance Policy Deliberative Committee during this month. In particular, it is noteworthy that a plan to increase the outpatient co-payment rate of minor patients, such as simple colds usin
- Policy
- Bioequivalence reevaluation may hinder drug license renewal
- by Lee, Tak-Sun Apr 1, 2020 06:21am
- Korean pharmaceutical industry is voicing their concerns about the Ministry of Food and Drug Safety’s (MFDS) plan to revive bioequivalence reevaluation and to toughen the item license renewal review procedure. Arguing the government’s redundant review could harm them, the industry claims the ministry should set down more consistent re
- Policy
- Coverage on COVID-19 treatments to last a year
- by Lee, Hye-Kyung Apr 1, 2020 06:20am
- Despite the expedited review, the health insurance reimbursement on the off-label drug would be reassessed a year after due to the COVID-19 outbreak. Korea’s Health Insurance Review and Assessment Service (HIRA) disseminated a press release on Mar. 30 and stated, “Taking in account the latest medical case studies and expert recommendation,
- Product
- 7 out of 10 doctors, the government was wrong with COVID-19
- by Kang, Shin-Kook Apr 1, 2020 06:17am
- Seven out of ten doctors found that the government's response to the COVID-19 was wrong. The Korean Medical Association (Chairman Dae-Zip Choi) conducted a questionnaire survey on the COVID-19 events through the doctoral survey on the 20th to 24th, and published the results on the 30th. As a result of the survey, 39.1% (621 people) of the
- Company
- GC LabCell to develop COVID-19 treatment using NK cell
- by Lee, Seok-Jun Apr 1, 2020 06:17am
- GC LabCell is developing a COVID-19 treatment using natural killer (NK) cell. The Korean company is tentatively planning to start a clinical trial in Korea and the U.S. later this year. On Mar. 30, GC LabCell announced the company and the U.S.-based biotech company KLEO Pharmaceuticals are collaborating on a research for the COVID-19 trea