- Company
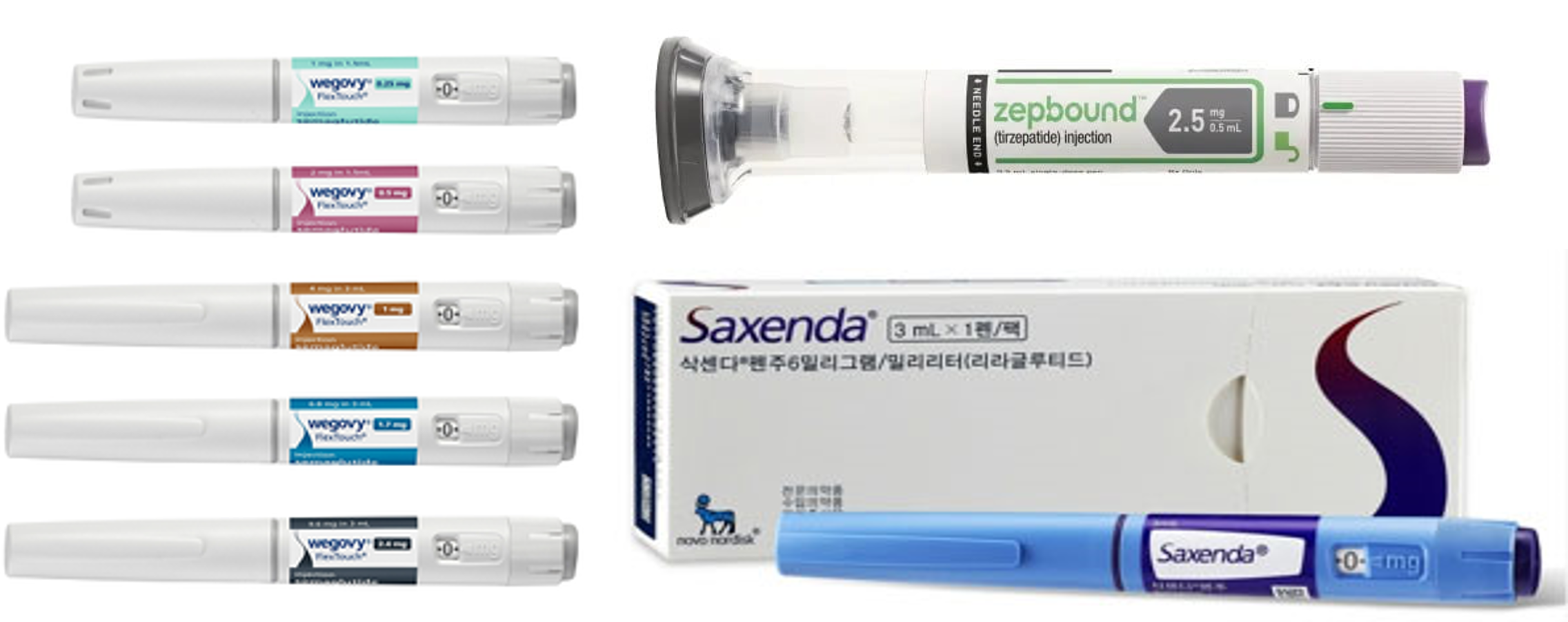
- Wegovy dominates the obesity drug market
- by Chon, Seung-Hyun Mar 13, 2025 05:59am
- The introduction of Novo Nordisk's Wegovy has shaken the market substantially. Since its launch in Q4, Wegovy has captured 60% of the entire market. Given the introduction of Wegovy, the obesity market expanded to the largest in history. It also consumed the market for Saxenda, containing the same class of active ingredients as Weogvy. It is unc
- Company
- "High hopes for Blincyto as consolidation therapy for ALL"
- by Whang, byung-woo Mar 13, 2025 05:58am
- Blincyto (blinatumomab), a treatment for acute lymphoblastic leukemia (ALL), is approved for an expanded indication as a consolidation therapy. Its role in clinical settings will be broadened. Experts view that having more treatment options is favorable, as the consolidation therapy option has been limited following the first-line treatment o
- Policy
- Mandatory reporting of market supply suspended drugs
- by Lee, Hye-Kyung Mar 13, 2025 05:58am
- The mandatory reporting is being considered to include drug products of suspended production·importation and supply, and 'supply shortage drugs' of which supply has been halted for more than 1 month. The Ministry of Food and Drug Safety (MFDS; Minister, Yu-Kyoung Oh) stated that it would announce on March 11 an administrative notice of revis
- Company
- ‘Keytruda’s 10-year milestones show its core value’
- by Whang, byung-woo Mar 13, 2025 05:58am
- Although many drugs have left their mark on the domestic pharmaceutical market, it is difficult to talk about the last decade without mentioning the immuno-oncology drug, Keytruda (pembrolizumab). As a drug that holds the title of the No. 1 in global sales, it has left various milestones in Korea, from sales to approved indications. Th
- Policy
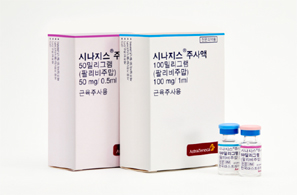
- Shortage of Synagis supply announced…normalized by May
- by Lee, Hye-Kyung Mar 13, 2025 05:57am
- With a shortage in the supply of Synagis (palivizumab), a preventive antibody for respiratory syncytial virus (RSV), which is a main cause of pneumonia and bronchiolitis in infants and young children, expected in Korea, concern arises on how the lack of supply may hinder disease prevention for children in high-risk groups. On the 11th, As
- Company
- Emergence of a next-generation lung cancer targeted drug?
- by Son, Hyung Min Mar 12, 2025 05:57am
- Clinical trials on 4th generation non-small-cell lung cancer targeted therapies by domestic and international pharmaceutical and biopharmaceutical companies are progressing smoothly. J INTS BIO, Voronoi, Therapex, and BridgeBio are continuing their development making clinical success. Among global pharmaceutical companies, Blue Diamond has enter
- Company
- New ADC for breast cancer 'Trodelvy' at the final reimb step
- by Eo, Yun-Ho Mar 12, 2025 05:57am
- A new antibody-drug conjugate (ADC) for breast cancer, 'Trodelvy,' will enter the last stage toward being added to the insurance reimbursement list. The Ministry of Health and Welfare (MOHW) ordered the National Health Insurance Service (NHIS) to begin drug pricing negotiations for Gilead Sciences Korea's triple-negative breast cancer (TNB
- Policy
- New types of risk-sharing agreements added for reimbursement
- by Lee, Tak-Sun Mar 12, 2025 05:57am
- The Health Insurance Review and Assessment Service has established two types of risk-sharing schemes, including initial treatment cost reimbursement (Fixed cost refund at initial treatment) and outcome-based reimbursement, to the detailed criteria for drugs subject to negotiation. This appears to reflect the additional content included in
- Company
- 12 Korean biosimilars globally approved in 3 months
- by Chon, Seung-Hyun Mar 12, 2025 05:56am
- Domestic pharma and biotech companies have achieved 12 biosimilar approvals in the U.S. and Europe this year. Celltrion and Samsung Bioepis have surpassed last year's record of 11 approvals in three months due to their rapid global expansion. Together, Celltrion and Samsung Bioepis have received over 20 biosimilar approvals in Europe and the Uni
- Opinion
- [Reporter’s View] Worth of ease in administration
- by Eo, Yun-Ho Mar 11, 2025 05:54am
- You can now orally take injectables, change the daily doses to monthly doses, and manage your disease with once a year injections. Such 'convenience of administration' has now become a competitive edge in the pharmaceutical market. Convenience, which had been mainly emphasized for chronic diseases, is now being highlighted in various oth