- 'Prostate cancer market leader' Astellas eyes on KRW 300 bil
- by Hwang, Byung-woo | translator Kang, Shin-Kook | Jul 19, 2024 05:48am
In addition to its leading product, Prograf (tacrolimus), showing strong sales, the company is expected to continue to generate sales growth when Padcev (enfortumab vedotin), an ADC for the treatment of urothelial cancer, become reimbursable.

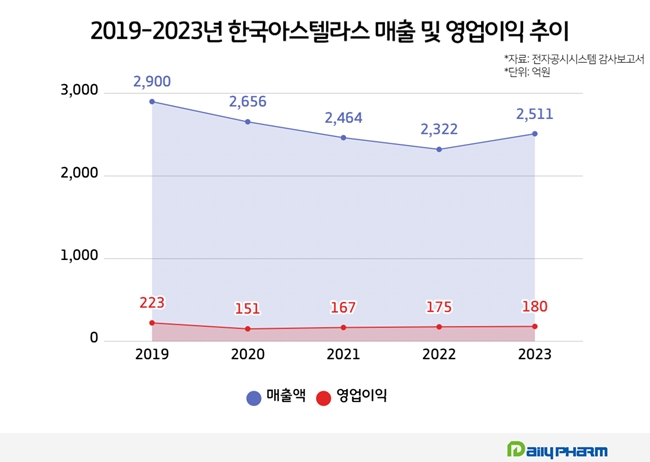
After sales peaked at KRW 290 billion in 2019, Astellas Pharma Korea experienced a decline to KRW 232.2 billion in 2022
Last year, Astellas Pharma Korea's sales amounted to KRW 251. 1 billion, up 7.5% from KRW 232. 2 billion in 2022. This marks a rebound within 4 years after 2019.
The company's sales peaked at KRW 290 billion in 2019, then declined every year with KRW 275.6 billion in 2020, KRW 246.4 billion in 2021, and KRW 232.2 billion in 2022.
Astellas Pharma Korea's operational profit in 2019 was KRW 22.3 billion, and it declined to KRW 15.1 billion in 2022. After that, it recovered to KRW 16.7 billion in 2021 and KRW 17.5 billion in 2022. Last year, its operational profit rose to KRW 19 billion.
Last year, Astellas Pharma Korea's selling and administrative expenses increased to KRW 80.9 billion from KRW 77.7 billion, up by KRW 13.2 billion. This rise included increased commission expenses (up by KRW 6 billion) and advertising and promotional expenses (up by KRW 2 billion). However, the company increased operating profit due to an overall increase in gross profit.
Astellas Pharma Korea's sales growth was brought about by its prostate cancer drug, Xtandi. It is an oral androgen receptor inhibitor (also known as ARTA). Xtandi's sales (according to IQVIA) increased from KRW 43.2 billion in 2023 to KRW 29.1 billion in 2022.
Xtandi's sales surpassed Erleada's KRW 15.9 billion and Xtandi's KRW 19 billion during the same period.
Such marked growth can be attributed to expanded reimbursement. In August 2022, Xtandi was selectively reimbursed for the treatment of patients with advanced prostate cancer accompanied by distant metastasis when used in combination with androgen deprivation therapy (ADT). Since November of last year, reimbursement has been made regardless of using other androgen synthesis inhibitors.
Xtandi is expected to generate further sales growth following expanded indication for the treatment of nonmetastatic, hormone-sensitive prostate cancer (nmHSPC).
With this approval, Xtandi has become the only ARTA that can be applied to all stages of prostate cancer stages following biochemical recurrence, including hormone-sensitive, castration-resistant, non-metastatic, and metastatic stages.
Prograf maintained KRW 90 billion range in sales for three consecutive years…sales growth expected for Xospata and Padcev
While Xtandi has shown significant growth among Astellas products, Prograf, the leading product, has shown a strong presence with sales in the KRW 90 billion range for three consecutive years.
Prograf is known for its indications in organ transplantation and immunosuppressive therapy. Generics were launched after the Prograf patent expired in 2005. However, Prograf still maintains over half of the market share in its ingredient segment.
Prograf's sales peaked at KRW 90 billion range for the first time in 2021, at KRW 91.5 billion, and it is still maintaining sales, generating KRW 90.6 billion in 2022 and KRW 91.4 billion in 2023. In addition, it continues to expand its presence in the competitive rheumatoid arthritis market.
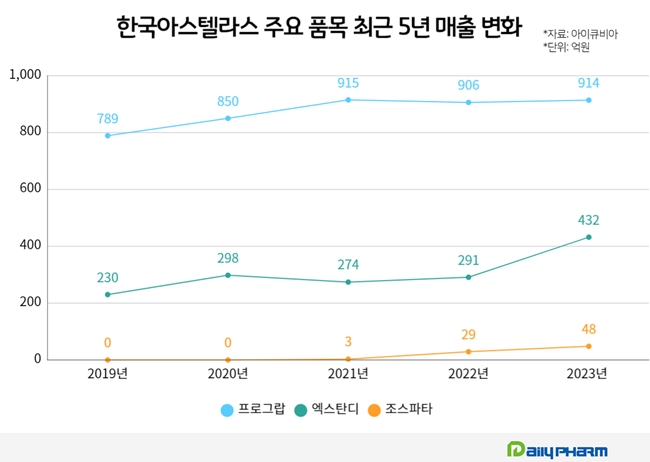
Last year, Xospata's sales amounted to KRW 4.8 billion, up 63% from KRW 2.9 billion in 2022. This year's sales are anticipated to increase further due to expanded National Health Insurance criteria in March of last year.
Initially, Xospata has been reimbursed with the National Health Insurance as a monotherapy in March 2022. However, it was reimbursed only for patients eligible for allogeneic hematopoietic stem cell transplantation, with a maximum limit of four cycles.
However, starting in March of this year, the limitation on eligibility for allogeneic hematopoietic stem cell transplantation and treatment duration have been lifted. As a result, Xospata is reimbursable for all adult patients with FLT3 mutation-positive relapsed/refractory acute myeloid leukemia, in accordance with domestic approval requirements.
With the removal of the previous restrictive reimbursement criteria, treatment access is expected to significantly improve for elderly patients who previously could not benefit from the medication and for those who had no treatment option due to ineligibility for allogeneic hematopoietic stem cell transplantation.
It is to be watched whether Astellas Pharma Korea will once again reach its previous highest sales of KRW 290 billion in 2019.
The pharmaceutical industry anticipates that Padcev, the company's new ADC prostate cancer drug, will drive future growth. In February, Padcev passed the Cancer Disease Review Committee (CDRC) of the Health Insurance Review and Assessment Service (HIRA) and has completed the economic evaluation. It remains to be seen when the drug will be considered for the Drug Reimbursement Evaluation Committee (DREC) review.
Padcev's sales for last year were only KRW 900 million, but it is already expanding its presence in the global market. When it becomes available with reimbursement, it has the potential to generate steep sales growth.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









