- Guidelines for new obesity drugs must be established
- by Moon, sung-ho | translator Kang, Shin-Kook | Jul 26, 2024 05:46am
Following this trend, pharmaceutical and biotech industry is focusing on developing next-generation treatments for obesity.
Glucagon-like peptide 1 (GLP-1) drugs, including Saxenda, Wegovy, and Zepbound, showed outstanding weight loss effects in clinical trials. As a result, companies are developing GLP-1-based therapeutics.
Recently, these drugs have been found to provide cardiovascular benefits and aid weight loss effects. As a result, their expanded use has been gathering attention in clinical settings. The analysis suggests that it's time to consider using these drugs in clinical settings in South Korea.
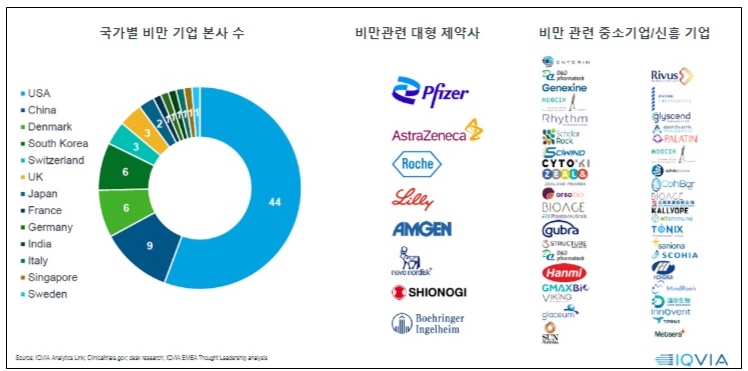
According to drug market research company IQVIA on July 20th, there are 79 obesity drug pipelines worldwide, from preclinical to launched products. Pharmaceutical and biotech companies have developed over 148 products.
GLP-1 drugs account for 39% of the pipelines. This suggests that pharmaceutical and biotech companies have begun developing drugs in this class as latecomers after witnessing the success of Saxenda, Wegovy, and Zepbound.
79 obesity drug pipelines worldwide, from preclinical to launched products, and 148 products from pharmaceutical and biotech companies have been developed.
The analysis indicates that major companies that have begun developing obesity drugs employ two major development strategies.
They either develop a drug as 'monotherapy' based on their differentiation strategy, or consider potential expansion for treating obesity, type 2 diabetes, cardiovascular diseases, and metabolic dysfunction-associated steatohepatitis (MASH) as part of their portfolio strategy.
As part of differentiation therapy, monotherapy is being developed to achieve the ▲Highest weight loss rate, ▲Improved safety, and ▲Chronic disease management with oral formulation. For instance, Pfizer and Viking Therapeutics are developing candidate products. Under the portfolio strategy, the market leaders, such as Novo Nordisk (Wegovy) and Lily (Zepbound), and latecomers, aim to expand indications.
Lately, the development of 'oral formulation drugs' has gained attention. These drugs are expected to shift the paradigm of a market dominated by injectables. Global companies, Pfizer and Viking Therapeutics, and domestic companies, including Ildong Pharmaceutical and D&D Pharmatech, have started to develop them. Novo Nordisk and Lily, the market-leading companies that already have injectables, have proprietary pipelines.
However, Wegovy and Zepbound, which dominate the global market for obesity, have unresolved issue of weight loss rebound.
IQVIA Korea's Marketing & Sales Director Kang-Bok Lee said, "Most obesity pipelines at the clinical stage are being developed as oral formulations." Lee added, "However, there are still discussions about oral obesity drugs. Along with the convenience, we must consider whether these drugs are suitable for chronic and maintenance management, and whether their cost and supply network outweigh any remaining issue."
Lee added that "There are questions about whether it can have similar efficacy compared to injectables, as well as concerns about tolerability." Lee expressed optimism about oral drug development, saying, "Recently, Viking's oral drug, VK2735, had no clinically significant gastrointestinal side effects compared to placebo, with most being mild."
Reflecting on the global trend for obesity drug development, new GLP-1 drugs, such as Wegovy and Zepbound, will likely be introduced to South Korea.
According to IQVIA, the market for obesity drugs is rapidly growing after the launch of Wegovy. The worldwide market size in 2023 totaled US$11 billion (about KRW 15.3 trillion), driven by Wegovy. Wegovy contributed 72% of the total US$11 billion-worth market in 2023.
In contrast, in South Korea, the release of Wegovy has been delayed due to an issue with 'securing stock' after obtaining marketing approval. As a result, Saxenda (Novo Nordisk) and Qsymia (Alvogen Korea) have taken 60% of the market share, dominating the market.
Sources said that the release of Wegovy is set to be released in the Korean market, making it the ninth country globally.
A professor from the Department of Endocrinology at an unnamed University Hospital, who is also an executive member of the Korean Society for the Study of Obesity, said, "Following Japan, China has also approved Wegovy. Since an official launch date has not yet been set, it is difficult to guarantee the timing of the release." He analyzed, "This appears to reflect the position of the domestic market within the global market."
"Even if it is released, it seems that obesity drugs will be used entirely as non-reimbursable in the domestic market," He added. "While there has been some progress in recognizing obesity as a disease, reimbursement coverage, especially concerning domestic insurance finances, will not be easy."
As a result, the pharmaceutical and biotech industries have proposed that, considering the potential of the drug market due to the rising prevalence of obesity, there is a need to accelerate discussions on disease recognition, enhancements in social awareness, and the establishment of clinical practice guidelines and insurance.
At the same time, domestic pharmaceutical and biotech companies developing treatments may need to focus on differentiation strategies from competing products, improving efficacy such as preventing weight regain after discontinuation and establishing a stable supply chain to meet global demand.
IQVIA Korea's Director Kang-Bok Lee said, "In the past two years, global spending on obesity has increased rapidly with new drugs, and by 2030, more than 15 new items are expected to enter the market, making the next-generation obesity treatment market much more competitive." Lee also said, "Improving educational programs for healthcare professionals to raise awareness of obesity treatment and integrating it into chronic disease management would be an ideal approach."
Lee also added, "Currently, even though obesity treatments are approved by the Ministry of Food and Drug Safety (MFDS), there are no cases where these drugs are covered by reimbursement. In the U.S., Medicare (Part D) will now cover Wegovy for some patients with a history of heart disease, as announced by the Centers for Medicare & Medicaid Services (CMS)." Lee added, "In the future, obesity drugs will be divided into reimbursed and out-of-pocket markets, so it is necessary to consider establishing reimbursement criteria for patients with severe obesity or accompanying diseases. We must develop and distribute comprehensive obesity treatment guidelines through collaboration with the medical community and organizations."
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









