- 'Xtandi and Erleada' compete in the prostate cancer market
- by Hwang, Byung-woo | translator Kang, Shin-Kook | Aug 14, 2024 05:51am
Astellas Pharma's Xtandi has increased its competitiveness with its indications, Janssen has started to face competition by expanding its portfolio, including Erleada, Zytiga, and Akeega.
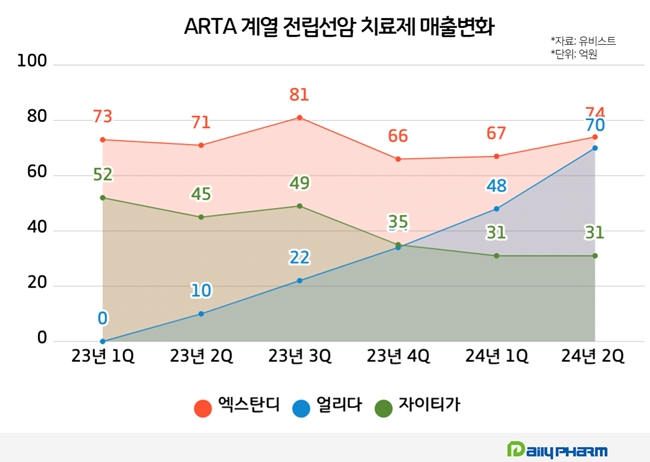
According to the pharmaceutical market research firm UBIST, Xtandi generated the highest outpatient prescription sales in this year's first half, KRW 14.1 billion.
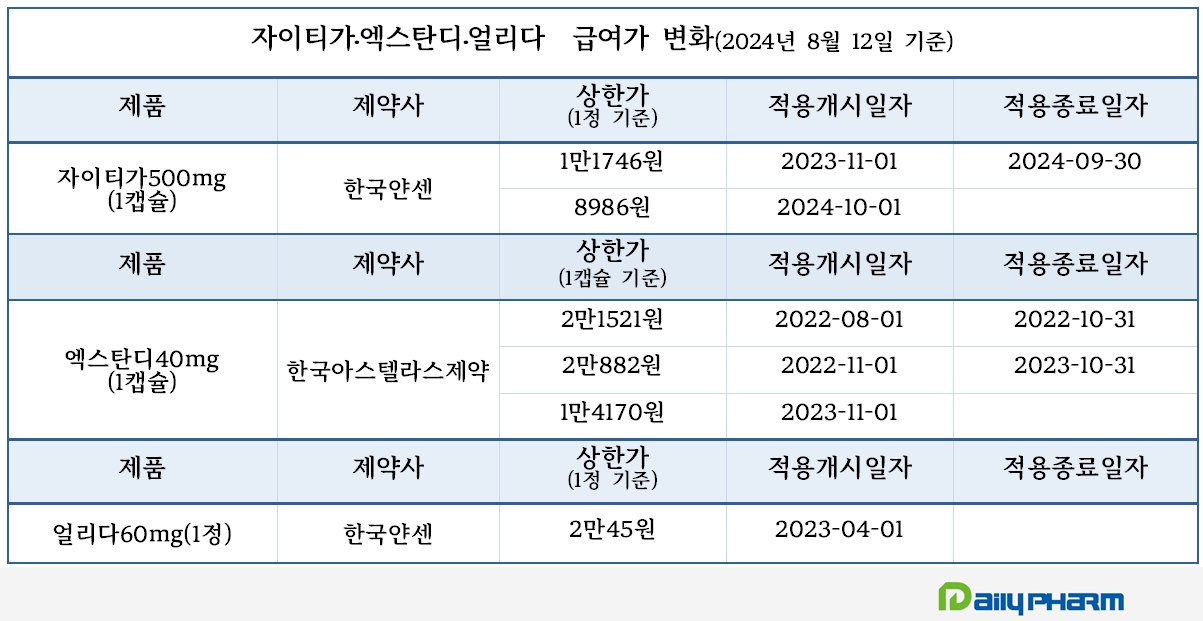
Analysis suggests that increased outpatient prescription sales are due to expanded reimbursement. In August 2022, Xtandi was selectively reimbursed for the treatment of patients with advanced prostate cancer accompanied by distant metastasis when used in combination with androgen deprivation therapy (ADT). Since November of last year, reimbursement has been made regardless of using other androgen synthesis inhibitors.
However, the drug price was reduced from KRW 20,882 to KRW 14,170 due to the expanded reimbursement range, affecting prescription sales. Xtandi's prescription sales for this year's Q3 was KRW 8.1 billion, then reduced to KRW 6.6 billion in Q4.
In November last year, the co-payment rate for Xtandi was adjusted from 30% to 5%, canceling out the prescription price reduction. Xtandi's sales recovered, with KRW 6.7 billion in this year's Q1 and KRW 7.4 billion in Q2.
Also, in June, Xtandi expanded its indication to non-metastatic hormone-sensitive prostate cancer (nmHSPC), which is expected to increase prescription sales in the future.
With the expanded indications, Xtandi became the only ARTA treatment that can be prescribed to treat all prostate cancer stages following biochemical recurrence, including hormone-sensitive, castration-resistant, non-metastatic, and metastatic.
An Astellas official said, "Xtandi is the only prostate cancer treatment that can be used to treat hormone-sensitive HSPC at early stages regardless of metastasis, non-metastasis, and the number of metastases." He said, "When the indication to treat nmHSPC was recently approved, the company focused on marketing to raise awareness of the importance of early ARTA combination therapy to treat HSPC."
Erleada chasing Xtandi…Janssen, "To provide patient-individualized treatment option"
Although Xtandi is the market leader, Janssen's Erleada, which has been reimbursed since last year, rapidly generates prescription sales.
Erleada generated prescription sales of KRW 3.4 billion in last year's Q4. Its sales grew with a bigger margin, with KRW 4.8 billion in this year's Q1 and KRW 7 billion in Q2. Its prescription sales in Q2 were close to Xtandi.
Analysis suggests that the close competition may be due to Xtandi's drug price reduction. Erleada appears to be established in clinical practice, as it has been a year since receiving reimbursement.
On the other hand, the prescription sales of Janssen's Zytiga (abiraterone) have decreased after the introduction of generic versions.
Last year, prescription sales for Xytiga decreased from KRW 4.9 billion in Q3 to KRW 3.5 billion in Q4. In this year's Q1 and Q2, it generated KRW 3.1 billion due to expanded prescriptions of competitor drugs and the introduction of generic products.
After Hanmi Pharm's Abiterone was approved last year, generic version of Xytiga were introduced to the market for Xytiga. Furthermore, Hanmi Pharm launched the combination drug Abiterone Duo in February, and Ace Pharmaceuticals obtained approval for Amaron. With more competitors, Xytiga's prescription sales are expected to decrease even more.
Janssen plans to focus on expanding treatment options for prostate cancer sub-types to compete against Xtandi, which has broad indications.
Janssen's prostate cancer treatment portfolio consists of Xytiga, Erleada, and Akeega. Akeega was approved in September of last year.
Akeega is a treatment for BRCA1/2 mutation-positive metastatic castration-resistant prostate cancer. It is a combination drug combining abiraterone and the PARP inhibitor ingredient niraparib.
Prescription sales of individual drugs may be lower than those of Xtandi, but the company's strategy is to face the competition by diversifying treatment options.
A Janssen official said, "For the treatment of prostate cancer in South Korea, we have been putting efforts to provide various individualized treatment options and improve treatment accessibility." "We will strive to work as a market leader to provide Korean patients with prostate cancer with optimized treatment by expanding treatment options through Erleada and Xytiga and expanding our portfolio, including Akeega.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.
- [Op-Ed] Patients, no time left for 'new drug comb therapies'
- Special Contribution | Eo, Yun-Ho









