- Enhertu redefines the standard in early-stage breast cancer
- by Hwang, byoung woo | translator Alice Kang | Oct 20, 2025 06:08am
Both the DESTINY-Breast05 and DESTINY-Breast11 trial results that were presented at the 2025 European Society for Medical Oncology (ESMO) Congress in Berlin showed significant results, positioning T-DXd as a potential new standard of care across both neoadjuvant (pre-surgical) and adjuvant (post-surgical) settings.

T-DXd reduces risk of recurrence by 53% compared to Kadcyla as postoperative adjuvant therapy
DESTINY-Breast05 is a Phase III head-to-head trial directly comparing Enhertu with the standard therapy Kadcyla (trastuzumab emtansine, T-DM1) in patients with HER2-positive early breast cancer who had residual invasive disease after neoadjuvant therapy (chemotherapy and targeted therapy before surgery).
Professor Dr. Charles E. Geyer of the University of Pittsburgh Medical Center (UPMC) Hillman Cancer Center, who presented the data at ESMO, emphasized, “In high-risk patients with residual disease, T-DXd demonstrated a clear survival benefit over T-DM1.”
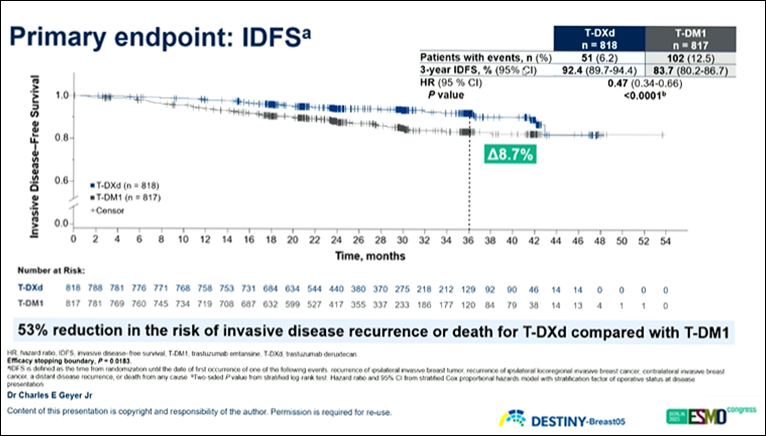
At the interim analysis, 3-year invasive disease-free survival (IDFS) was 92.4% for T-DXd (95% CI 89.9–94.4) versus 83.7% for T-DM1 (80.2–86.7), reflecting a 53% reduction in risk of events (HR 0.47, p<0.0001).
Similarly, disease-free survival (DFS) was 92.3% vs 83.5%, distant recurrence-free interval (DRFI) was 93.9% vs 86.1% (HR 0.49), and brain metastasis-free interval (BMFI) reached 97.6% vs 95.8%.
The 3-year overall survival (OS) rate was also higher with T-DXd (97.4% vs 95.7%), showing early separation of survival curves even at the early breast cancer stage.
Pressor Geyer noted, “Most ILD cases were mild and reversible with early recognition and intervention. With appropriate monitoring and management, ILD is fully manageable.”
He concluded, “T-DXd has demonstrated clear benefits in terms of both IDFS and DFS for high-risk HER2-positive patients and has the potential to replace T-DM1 as a new standard of care.”
# Also shows superiority as preoperative neoadjuvant therapy… “T-DXd-THP is safer and stronger”
The DESTINY-Breast11 (291O) trial, also announced that day, compared standard ddAC-THP with T-DXd monotherapy (8 cycles) or T-DXd (4 cycles) followed by paclitaxel, Herceptin (trastuzumab), and Perjeta (pertuzumab) combination therapy (T-DXd-THP).

Consistent superiority was confirmed in both hormone receptor-positive (HR+) patients (61.4% vs 52.3%) and hormone receptor-negative (HR–) patients (83.1% vs 67.1%).
Regarding safety, Grade ≥3 adverse events were lower in T-DXd-THP (37.5%) vs ddAC-THP (55.8%), and left ventricular dysfunction was 1.9% vs 9.0%. Drug-related ILD was comparable at 4.4% vs 5.1%.
Professor Nadia Harbeck of Ludwig Maximilian University Hospital, Munich, who presented the data, explained, “T-DXd-THP demonstrated a statistically significant improvement in pCR compared to standard therapy with lower toxicity. It could become a compelling option for anthracycline-free neoadjuvant therapy.”
“Discussion on timing of use—before vs. after surgery—will begin”
Dr. Sara Tolaney of Dana-Farber Cancer Institute commented that, “T-DXd retains the same antibody backbone as T-DM1 but incorporates a more potent payload and bystander effect, representing a key structural evolution.”
She added, “Despite including a higher-risk population than the historic KATHERINE trial, DESTINY-Breast05 achieved significant survival improvements, underscoring its strong clinical relevance.”
Experts anticipate that the timing of T-DXd administration will become a key point of discussion in future clinical practice.

At the congress, Professor Yeon-Hee Park, Professor of Hematology-Oncology at Samsung Medical Center, said, “Enhertu has already been an established therapy in metastatic breast cancer, but these new DESTINY-Breast05 results demonstrate its significant efficacy even in early-stage disease. In Korea, discussions on incorporating it into clinical practice for post-surgical residual disease or high-risk patients will likely proceed rapidly.”
She added, “Although DESTINY-Breast11 has interpretive limitations due to its design, it opens new discussions on toxicity management and combination strategies. Enhertu (T-DXd) could demonstrate greater clinical value in patient populations where Kadcyla (T-DM1) fell short.”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.
- [Op-Ed] Patients, no time left for 'new drug comb therapies'
- Special Contribution | Eo, Yun-Ho









