- AstraZeneca seeks mutual growth through open collaboration
- by Hwang, Byung-woo | translator Kang, Shin-Kook | Aug 27, 2024 05:50am

Multinational pharmaceutical companies are increasingly investing in R&D in Korea’s domestic pharmaceutical industry through open innovation.
According to the Korean Research-based Pharmaceutical Industry Association (KRPIA), the total amount of R&D invested in clinical research in Korea in 2022 was KRW 817.8 billion and has been on the rise for 3 consecutive years.
AstraZeneca Korea, which recently introduced bold new drugs such as Enhertu and Imfinzi, is also aiming to create a virtuous cycle of shared growth with the domestic pharmaceutical industry through active R&D investment.
AZ invests more than 30% of sales in R&D...makes notable achievements in developing innovative drugs
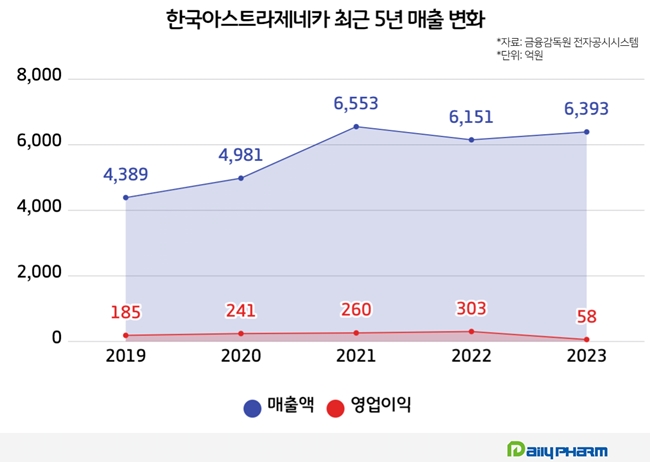
AstraZeneca Korea's sales surpassed the KRW 600 billion mark in 2021 (KRW 655.3 billion), based on audited financial statements.
At the time, sales were boosted by its COVID-19 vaccine, but the company's robust anti-cancer portfolio and rare disease therapies acquired through the acquisition of Alexion have since become new growth drivers, posting sales of KRW 615.1 billion in 2022 and KRW 639.3 billion in 2023.
AstraZeneca Korea's sales growth is significant because the company has invested more than 30% of its revenue back into the domestic industry.
According to the company, it invested KRW 215 billion in Korea in 2023, which is about 34% of its annual revenue. Of this, KRW 116 billion was spent on clinical research.
This coincides with the discussions it had made at the Korea-Sweden Business Summit that was held in Stockholm, Sweden in 2019. At that time, AstraZeneca announced a plan to invest KRW 850 billion in Korea, which it implemented for 5 years.
AstraZeneca's investment in Korea is particularly noteworthy because it is not limited to R&D.
For example, the global clinical TOPAZ-1 trial of the immuno-oncology drug Imfinzi, which was led by Dr. Do-Yoon Oh, professor of Medical Oncology at Seoul National University Hospital, changed the global biliary tract cancer treatment paradigm by showing the potential to improve survival outcomes in biliary tract cancer, a disease with an average survival period of less than one year with existing treatments.
In 2023, the company co-developed and launched Sidapvia, a combination diabetes drug, after 4 years of collaboration with SK chemicals, and is currently working together for its global commercialization.
AstraZeneca Korea is also expanding its collaboration with the Korean government to strengthen domestic research capabilities. The company has been running the ‘KHIDI-AZ Anti-Cancer Research Support Program’ for over a decade, a program that selects and supports research projects in the field of anti-cancer with the Korea Health Industry Development Institute (KHIDI), with the goal of overcoming cancer, the No.1 cause of death in Korea.

Four of the projects that have been completed under the program have led to tangible results, including the publication of▲SCI papers, ▲lectures at the official international conferences of the Korean Diabetes Association and the Asian Association for the Study of Diabetes, and ▲publications in the Journal of the Korean Diabetes Association.
As a result of these open innovation achievements, the company has been selected as an 'innovative pharmaceutical company accredited by the Ministry of Health and Welfare' for 6 consecutive years since 2018. As of June, there are only 3 multinational pharmaceutical companies that fall in the innovative pharmaceutical company category as announced by the Ministry of Health and Welfare.
Aims to create a patient-centered care ecosystem and rebuild community for development
Ultimately, AstraZeneca Korea believes that beyond the development and supply of innovative medicines, it is necessary to address the blind spots in treatment that occur in the pre-approval and reimbursement stages.
Examples include attracting clinical trials to Korea so that patients can start treatment with innovative medicines that are not yet available in the country, and running patient support programs to increase patient access to medicines that are yet to be reimbursed in Korea.
As of 2023, AstraZeneca Korea is conducting approximately 130 clinical trials in Korea, making it the pharmaceutical company with the most clinical trials approved by the Ministry of Food and Drug Safety (MFDS), excluding CROs.
Through AstraZeneca's clinical trials, about 2,600 cancer patients in Korea have received new anti-cancer drugs in the last 5 years (2018-2023), and about 1,004 patients with ultra-rare diseases have been treated through a total of 37 clinical trials in the last 6 years (2019-2024).
The company also continuously expands support to minimize treatment gaps. Currently, more than 10 early access programs are in place, and it is known that about 260 cancer and extremely rare disease patients have received treatment through these programs so far.

“AstraZeneca is committed to the development and supply of innovative medicines through increased investment in Korea, including in R&D talent,” said Sewhan Chon, Country President of AstraZeneca Korea. ‘We are constantly striving to create a healthcare ecosystem that can mutually grow with our community across the pharmaceutical industry, medical research, and patients’ lives.”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.
- [Reporter’s View] ‘Selection & Focus’ to foster K-Bios
- Reporter's view | Hwang, byoung woo









