- "Early intervention" needed for treating multiple myeloma
- by Whang, byung-woo | translator Hong, Ji Yeon | Oct 29, 2024 05:49am
Expert opinions indicate that the Korean medical treatment field changes with new drug approvals and reimbursement listings, yet patient access remains restricted.

According to the Health Insurance Review and Assessment Service (HIRA), the number of Korean patients with multiple myeloma is on the rise due to the aging population. Multiple myeloma patients were 7,063 in 2017, and the number increased to 11,219 patients in 2023.
"The average multiple myeloma onset is 60 years or higher. As the aging population continues to increase, the number of multiple myeloma patients will likely increase as well," Dr. Min explained.
The problem is that remission of multiple myeloma is difficult to achieve. Despite undergoing treatments, patients are likely to experience relapse. Therefore, treatment focuses on selecting effective treatments in each stage and extending the progression-free survival period.
Typically, treatment duration shortens as the number of treatments increases. For example, 95% of patients undergoing first-line treatment relapse, 15% of patients undergoing fourth-line treatment relapse, and the percentage drops to 1% by the time patients receive fifth-line treatment.
"When multiple myelomas relapse after several years following treatment at an early stage, the disease becomes chronic. It is important to undergo effective treatment at early stage so that the disease does not advance to secondary, tertiary, etc," said Dr. Min.
As new drugs are being developed for treating multiple myeloma, a variety of treatment options are available.
Originally starting from simple chemotherapy, the treatment evolved to combination therapies, including proteasome inhibitors, immune modulators, and high-dosage steroids. Recently, new treatments, such as anti-CD38 monoclonal antibodies and BCMA-targeted immune therapy, became available.
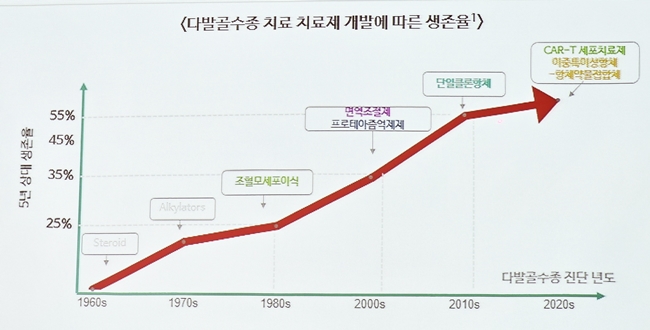
In South Korea, new treatment options are emerging as new drugs for treating multiple myeloma receive the Ministry of Food and Drug Safety (MFDS) approvals: elranatamab in May and Talvey in June.
However, the limitation to utilizing such treatments is that the National Health Insurance reimbursement is limited.
A 'DVTd combination therapy (Darzalex+bortezomib+thalidomide+dexamethasone),' containing Darzalex (daratumumab), recently received an appropriateness decision from the Drug Reimbursement Evaluation Committee (DREC) for expanding reimbursement as the first-line treatment of multiple myeloma. However, analysis suggests that the medical field still needs overall improvement.
"Within the limited government fund, many pharmaceuticals are covered by reimbursement. Yet, there still needs improvement based on the global standard," Dr. Min said. "Despite having good treatment options, including existing medications and new drugs, patient access is limited. Thus, it requires improvement."
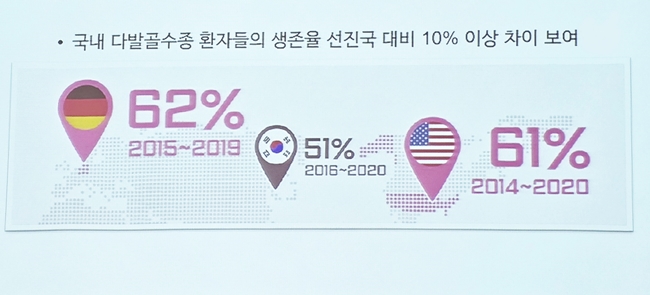
The survival rate of Korean patients with multiple myeloma is 51%, showing a 10% difference compared to that of advanced countries, 61% in the United States and 62% in Germany.
Consequently, the Korean medical field is changing due to new drug approvals and reimbursement listings. Various approaches may be needed to improve treatment outcomes, such as considering treating the disease at an early stage and optimizing the order of treatments.
Dr. Min has suggested an improvement plan as ▲Early use of the monoclonal antibody combination therapy ▲Triple combination drug that can be used in patients who are non-responsive to Lenalidomide ▲A new class treatment for patients relapse or refractory to over third-line therapies.
It suggests that administering a new drug that would significantly improve the survival rate at an early stage could achieve both the treatment effects and the National Health Insurance finance.
"We understand the problem of limited National Health Insurance finance, but we expect that early administration of an effective treatment method would reduce relapse rate and increase the survival rate, thereby potentially reducing the treatment cost," Dr. Min said. "Most patients currently undergo 6th and 7th treatments. Using effective treatments at an early stage will, in turn, be effective for the National Health Insurance."
Lastly, Dr. Min added, "It would be difficult for patients to fully experience the treatment effects when effective medications are used in patients with poor conditions after the 3rd and 4th treatments. By administering treatments early, we expect to see the global standard therapy results."
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









